Dear Editor,
World Health Organization (WHO) first declared coronavirus disease 2019 (COVID-19) as a pandemic in March 2020, and now, six months after that, the number of positive and death cases are still increasing. This global pandemic has caused a significant impact on health, social, and economic aspects around the world. Thus, identification of the risk factors that contribute to the development of severe infections is important to enabling risk stratification, optimizing the hospital resources reallocation, and guiding public health recommendations and interventions. Several medications have been demonstrated to be associated with a reduction in poor outcomes from COVID-19 such as anticoagulant and metformin, while other medications did not alter outcomes of COVID-19 infections such as ACE inhibitors, angiotensin II receptor blocker (ARB), and statin [1], [2], [3]. During normal times, proton pump inhibitors (PPIs) are among the drugs which are most commonly used by patients because of their efficacy in relieving dyspepsia and GERD symptoms, also because of their relatively affordable price [4]. In a previous meta-analysis study, it has been shown that the use of proton pump inhibitors (PPIs) may increase the risk of pneumonia even though the heterogeneity of the study is high [5]. Unfortunately, until now, the evidence regarding the link between the use of PPI and COVID-19 outcomes is still conflicting. This article aims to give better evidence for the association between PPI usage and in-hospital outcomes (severity and mortality) of COVID-19 infection.
A search of the literature was conducted on Google scholar using the keywords “proton pump inhibitors” OR “PPI” OR “clinical characteristics” OR “medications” OR “risk factors” AND “coronavirus disease 2019” OR “COVID-19”, between 2019 and present time (September 10th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of PPI usage in COVID-19 patients with a clinically validated definition of “severe disease” and “mortality” were included in this meta-analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles.
A meta-analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. Dichotomous variables were calculated using the Mantel-Haenszel formula with random-effects models. The heterogeneity was assessed by using the I 2 statistic with a value of < 25%, 26–50%, and > 50% were considered as low, moderate, and high degrees of heterogeneity, respectively. The effect estimate was reported as risk ratio (RR) along with its 95% confidence intervals (CIs) for dichotomous variables, respectively. P-value was two-tailed, and the statistical significance was set at ≤ 0.05.
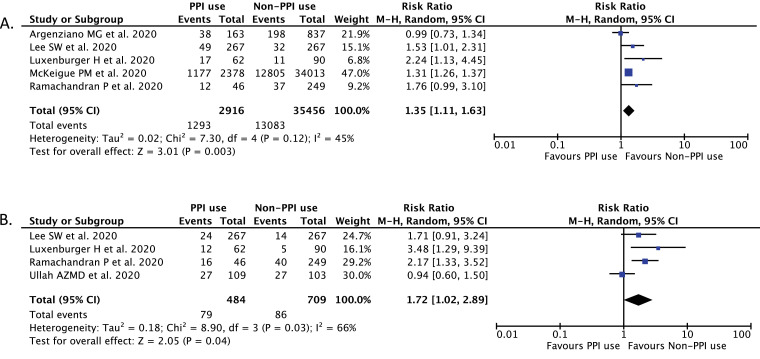
A total of 7300 records were obtained through systematic electronic searches and other ways. After screening titles, abstracts, and full texts, 6 studies [6], [7], [8], [9], [10], [11] with a total of 5884 COVID-19 patients were included in the meta-analysis (Table 1 ). The individual and pooled RRs for the associations between the use of PPI and severe outcome of COVID-19 is shown in Fig. 1 A, while the individual and pooled RRs for the association between PPI usage and mortality from COVID-19 is shown in Fig. 1B. Our pooled analysis showed that PPI usage is significantly associated with an increased risk of severe COVID-19 [RR 1.35 (95% CI 1.11–1.63), p = 0.003, I 2 = 45%, random-effect modeling] and mortality from COVID-19 infection [RR 1.72 (95% CI 1.02–2.89), p = 0.04, I 2 = 66%, random-effect modeling].
Table 1.
Characteristics of included studies.
| Study | Sample size | Design | Outcome | Age (years) | PPI usen (%) | Non-PPI usen (%) |
|---|---|---|---|---|---|---|
| Argenziano et al. [6] | 1000 | Retrospective cohort | Severity | 62.6 ± 18.5 | 163 (16.3%) | 837 (83.7%) |
| Lee et al. [7] | 534 | Retrospective cohort | Severity and Mortality | 48 ± 19.7 | 267 (50%) | 267 (50%) |
| Luxenburger et al. [8] | 152 | Retrospective cohort | Severity and Mortality | 65 ± 17 | 62 (40.7%) | 90 (59.3%) |
| McKeigue et al. [9] | 36,391 | Case-control | Severity | N/A | 13,982 (38.4%) | 22,409 (61.6%) |
| Ramachandran et al. [10] | 295 | Retrospective cohort | Severity and Mortality | 66.9 ± 14.2 | 46 (15.5%) | 249 (84.5%) |
| Ullah et al. [11] | 212 | Retrospective cohort | Mortality | 67.1 ± 19.7 | 109 (51.4%) | 103 (48.6%) |
Fig. 1.
Forest plot that demonstrates the association of PPI use with severe COVID-19 disease (A) and mortality from COVID-19 (B). Events mean severe outcomes or mortality of the disease.
Based on our pooled analysis of available data, the use of proton pump inhibitors (PPIs) seems to be associated with an enhanced risk of severity and mortality from COVID-19 infection. Several reasons can be proposed to explain this result. First, PPI exerts its effects by inhibiting the proton pump which results in the suppression of gastric acid production [12]. This profound hypochlorhydria can diminish the protective effect of gastric acid. As we know, the ACE2 receptor is also expressed in the mucosa of the gastrointestinal (GI) tract and the fecal-oral route has been raised as one of the potential modes of transmission for COVID-19. Therefore, suppression of gastric acid may increase the survival of SARS-CoV-2 in the stomach and increase the ability of the virus to invade the GI epithelial cells. This condition can increase the viral load which in turn results in a higher chance of developing cytokine storm and severe outcome of the disease [13]. Moreover, the profound hypochlorhydria condition can also cause an increase in gastric microbiota and small intestinal bacterial overgrowth. The resulting dysbiosis conditions might increase the likelihood of developing enteric infections and sepsis which could complicate the disease. Not only that, when micro-aspiration happened, the bacteria will also then colonize in the lung and develop the secondary infection. The secondary infection may increase the likelihood of developing Acute Respiratory Distress Syndrome (ARDS) and severe outcome of the disease which may increase the mortality rate from COVID-19 [14]. Besides hypochlorhydria, PPI can also cause several serious adverse events such as gastric tumors, cardiovascular disease, and nephrotoxicity. PPI can inhibit the enzymatic activity of dimethylarginine dimethylaminohydrolase (DDAH) which will inhibit the nitric oxide synthase with the promotion of inflammation and thrombosis, resulting in the development of the cardiovascular disease. The idiosyncratic effect of PPIs on the kidneys will also lead to recurrent acute interstitial nephritis, a humoral- and cell-mediated hypersensitivity reaction which results in inflammation of the renal interstitium and tubules. All of these adverse events from PPI can also contribute to the development of severe outcome and mortality from COVID-19 infection [15,16]. Finally, PPI can also modulate the immune response by inhibiting neutrophil function. Neutrophil plays a significant role in the innate immune system which is the first-line defense of the body to fight infection. Inhibition of neutrophil function may impair the ability of the body to eradicate the infection and may increase the severity of infection, including COVID-19 infection [17].
The limitation of this study is that the presence of confounding factors such as patients' age and comorbid conditions that can affect the relationship between PPI use and in-hospital outcome from COVID-19 should still be considered. Moreover, most of the included studies did not mention the information about the type, dose, duration, frequency, and compliance for PPI use. The global RR values of this meta-analysis were also lower than 2.0 which reduces the clinical meaning of the association found. Finally, most of the included studies in this meta-analysis are retrospective cohort design which has limited scientific strength and can only be used to establish an association, but not a causality between PPI use and severity or mortality from COVID-19 [15,16]. However, with this study, we hope that PPI usage can further be considered as an important factor in COVID-19 patients.
Hence, patients should be more careful when using proton pump inhibitors and only use it if there is a prescription from the doctors. Patients with a history of PPI use should take extra care to minimize the risk of infection from COVID-19. Physicians should be more cautious when prescribing proton pump inhibitors to patients, especially patients with COVID-19, and may consider giving histamine-2 receptor antagonist (H2RA) or antacid instead of PPI for the symptoms of dyspepsia which is not severe. For other indications besides dyspepsia, physicians should weigh the potential risk and benefit in each patient when prescribing PPI and may seek alternative treatment. Physicians should also take close monitoring for patients with COVID-19 who have a history of PPI usage to prevent the development of severe outcome and mortality from the disease. Finally, the use of PPI should be regarded as an important factor in future risk stratification models for COVID-19.
Funding
None.
Conflict of interest
None declared.
Acknowledgment
None.
References
- 1.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19 doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(5):983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(6):1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moayyedi P., Delaney B.C., Vakil N., Forman D., Talley N.J. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127(5):1329–1337. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Wang C.H., Li C.H., Hsieh R., Fan C.Y., Hsu T.C., Chang W.C., et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18(3):163–172. doi: 10.1080/14740338.2019.1577820. [DOI] [PubMed] [Google Scholar]
- 6.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.W., Ha E.K., Yeniova A.O., Moon S.Y., Kim S.Y., Koh H.Y., et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2020 doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 8.Luxenburger H., Sturm L., Biever P., Rieg S., Duerschmied D., Schultheiss M., et al. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor? J Intern Med. 2020 doi: 10.1111/joim.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeigue P.M., Kennedy S., Weir A., Bishop J., McGurnaghan S.J., McAllister D., et al. Associations of severe COVID-19 with polypharmacy in the REACT-SCOT case-control study. medRxiv. 2020 doi: 10.1101/2020.07.23.20160747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran P., Perisetti A., Gajendran M., Jean-Louis F., Bansal P., Dwivedi A.K., et al. Prehospitalization proton pump inhibitor (PPI) use and clinical outcomes in COVID-19. medRxiv. 2020 doi: 10.1101/2020.07.12.20151084. [DOI] [PubMed] [Google Scholar]
- 11.Ullah A.Z.M.D., Sivapalan L., Chelala C., Kocher H.M. COVID-19 in patients with hepatobiliary and pancreatic diseases in East London: a single-centre cohort study. medRxiv. 2020 doi: 10.1101/2020.09.07.20189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin J.M., Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner J. Fecal-oral transmission of COVID-19: could hypochlorhydria play a role? J Med Virol. 2020 doi: 10.1002/jmv.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corsonello A., Lattanzio F., Bustacchini S., Garasto S., Cozza A., Schepisi R., et al. Adverse events of proton pump inhibitors: potential mechanisms. Curr Drug Metab. 2018;19(2):142–154. doi: 10.2174/1389200219666171207125351. [DOI] [PubMed] [Google Scholar]
- 15.Savarino V., Marabotto E., Furnari M., Zingone F., Zentilin P., Savarino E. Latest insights into the hot question of proton pump inhibitor safety - a narrative review. Dig Liver Dis. 2020;52(8):842–852. doi: 10.1016/j.dld.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Savarino V., Dulbecco P., Savarino E. Are proton pump inhibitors really so dangerous? Dig Liver Dis. 2016;48(8):851–859. doi: 10.1016/j.dld.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Namazi M.R., Jowkar F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J Clin Pharm Ther. 2008;33(3):215–217. doi: 10.1111/j.1365-2710.2008.00907.x. [DOI] [PubMed] [Google Scholar]