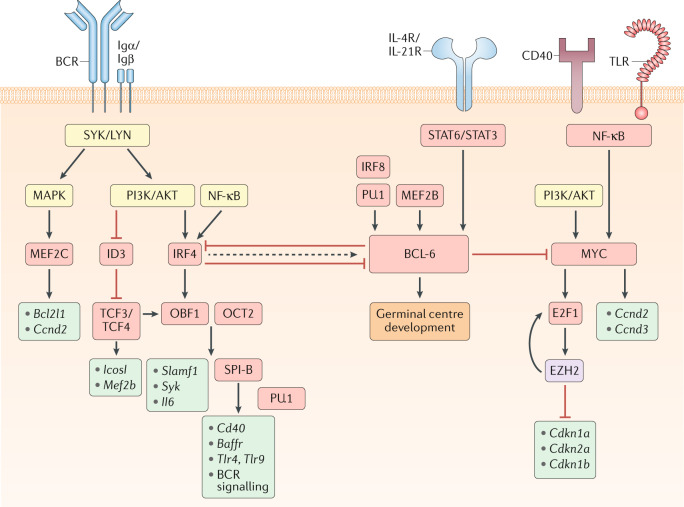
Fig. 1. Transcriptional regulation of GC B cell commitment.
Model for signalling pathways and transcription factors that regulate B cell commitment to the germinal centre (GC) fate. Boxes that indicate signalling molecules are coloured yellow, transcription regulators red, downstream gene targets turquoise and epigenetic modifiers purple. The B cell receptor (BCR), via its signalling subunits Igα and Igβ as well as downstream tyrosine kinases such as SYK and LYN, activates the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT pathways. MAPK signalling induces the expression of the transcriptional activator myocyte enhancer binding factor 2c (MEF2C), which promotes the transcription of B cell lymphoma-extra large (Bcl2l1) and cyclin D2 (Ccnd2). The expression of the transcriptional repressor inhibitor of DNA binding 3 (ID3) is reduced following B cell activation, allowing for transcription factor 3/4 (TCF3/4)-driven induction of Icosl and Mef2b transcription. PI3K/AKT and nuclear factor-κB (NF-κB) signalling also induce the expression of the transcription factor interferon regulatory factor 4 (IRF4). IRF4 and TCF3/4 induce the expression of the co-activator OBF1 (also known as POU class 2 homeobox associating factor 1 (POU2AF1)), which cooperates with OCT2 (also known as POU class 2 homeobox 2 (POU2F2)) to promote transcription of Slamf1, Syk and Il6. OBF1 and OCT2 can also induce the expression of the transcription factor SPI-B, which acts in a redundant fashion with PU.1 to enhance the transcription of genes encoding B cell surface receptors, such as Cd40, B cell activating factor receptor (Baffr), Toll-like receptor 4 (Tlr4) and Tlr9, and many components of the BCR signalling pathway, including Blnk and Btk. Although transiently elevated levels of IRF4 can induce the expression of B cell lymphoma 6 (BCL-6), sustained IRF4 levels will repress BCL-6 expression. BCL-6 expression is also induced by the transcription factors MEF2B and IRF8/PU.1 as well as the cytokines IL-4 and IL-21, which bind to their respective receptors (IL-4R and IL-21R) and induce signal transducer and activator of transcription 6 (STAT6)/STAT3 signalling. CD40 and/or TLR-driven NF-κB signalling, alongside PI3K/AKT signalling, will induce the expression of the transcription factor MYC, which promotes cellular proliferation by inducing the transcription of Ccnd2/Ccnd3 and the expression of the transcription factor E2F transcription factor 1 (E2F1). E2F1 induces expression of Ezh2 that encodes a Polycomb repressive complex 2 (PRC2) enzymatic component. Enhancer of zeste homologue 2 (EZH2) promotes cell cycle progression by repressing the expression of Cdkn1a, Cdkn2a and Cdkn1b, which encode cyclin-dependent kinase inhibitors. EZH2 also promotes E2F1 release from the retinoblastoma (Rb) protein via phosphorylation of Rb, thereby enhancing E2F1 activation and further EZH2 expression. BCL-6 can directly repress MYC expression, thereby limiting the number of cell divisions that GC B cells undergo. BCL-6 promotes GC B cell development through regulation of numerous genes controlling cellular processes including the DNA damage response, B cell migration, apoptosis, BCR and CD40 signalling, plasma cell differentiation and T cell:B cell interactions. Together, these transcriptional regulators allow for the precise control of GC initiation that is necessary to balance the competing needs of the immune system to induce a protective response while limiting immunopathology.