Metabolic acidosis is common in patients with chronic kidney disease (CKD) and may contribute to progression of CKD and all-cause mortality [1]. However, little is known about how CKD changes the response to an acute acid load and whether an altered response could contribute to adverse outcomes [2]. Therefore, our aim was to characterize the differences between the response to an acute oral acid load (to mimic the dietary acid load) in patients with CKD and healthy subjects.
To do so, we performed the short acid-loading test with ammonium chloride in 9 males with CKD Stage G4 and in 16 healthy male subjects (see Supplementary data for complete methods and baseline characteristics) [3]. The study was approved by the Medical Ethics Committee (MEC-2016-329) and registered at ClinicalTrials.gov (NCT03293446). In patients with CKD, all antihypertensive drugs (except β-blockers) were discontinued for 2 weeks to avoid drug interference. The test started after an overnight fast by giving a 10% oral solution of ammonium chloride (100 mg/kg body weight) over a period of 1 h together with a standardized meal (25 mmol sodium, 28 mmol potassium) and a water load (5 mL/kg followed by 2.5 mL/kg/h). The response to the acid load was observed for 6 h with repeated sampling of venous blood (at time 0, 3 and 6 h) and urine (hourly). Group comparisons were performed using repeated-measures two-way analysis of variance.
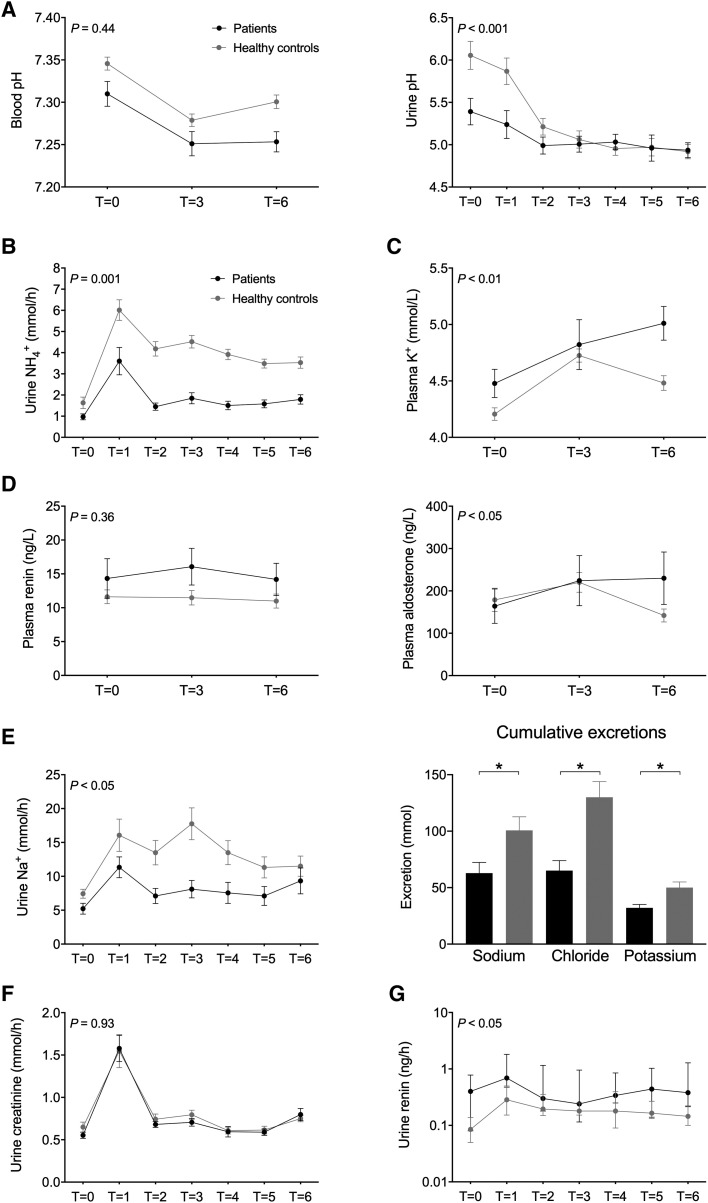
At baseline, blood and urine pH and plasma bicarbonate of the patients with CKD were significantly lower (Figure 1 and Supplementary data, Figure S1). After 3 h, ammonium chloride decreased blood pH and plasma bicarbonate and increased plasma potassium and plasma aldosterone similarly in both groups (Figure 1 and Supplementary data, Figure S1). At the end of the test, however, blood pH, plasma potassium and plasma aldosterone were returning to normal in the healthy subjects but not in the patients. Plasma renin did not change significantly during the test. The patients and healthy subjects adequately lowered urine pH (<5.3 in all participants). The healthy subjects and the patients also increased urine ammonium excretion after 1 h, but the increase in healthy subjects was significantly greater and persisted over time (Figure 1). This resulted in a significantly lower cumulative ammonium excretion in patients (Supplementary data, Figure S1). Accordingly, net acid excretion was also significantly lower in patients (no difference in titratable acid; Supplementary data, Figure S1). The same pattern as for urine ammonium excretion was also observed for urine sodium, chloride and potassium excretion. The urine excretion of creatinine, albumin and the low molecular weight proteins retinol-binding protein and renin also acutely increased after the acid load, with normalization thereafter (Figure 1 and Supplementary data, Figure S2). No differences between the groups were observed for the courses in creatinine and protein excretion, except for urinary renin. In patients with CKD, systolic blood pressure fell during the first 2 h and increased thereafter, whereas it remained stable in the healthy subjects (Supplementary data, Figure S2). Because patients were significantly older than healthy subjects, we also performed a subanalysis with older healthy subjects and found similar results (data not shown).
FIGURE 1.
Effects of an acute acid load with ammonium chloride on (A) venous blood pH and urine pH, (B) urine ammonium excretion, (C) plasma potassium (K+), (D) plasma renin and aldosterone, (E) urine sodium excretion and cumulative excretion of sodium, chloride and potassium, (F) urine creatinine excretion and (G) urine renin excretion. Group comparison was performed using repeated measures two-way analysis of variance reporting the P-value for interaction. Cumulative excretions were compared with unpaired t-tests. Urine renin was not normally distributed and was therefore log-transformed for analysis.
Here we characterized the response to an acute acid load on acid–base, electrolyte, creatinine and protein handling by the kidney and addressed whether this response is altered in patients with CKD. We showed that urinary ammonium excretion is reduced in patients with CKD, increasing the duration of the acidosis. Of note, per-nephron ammonium excretion was likely higher in patients with CKD, although this was not sufficient to prevent the acidosis after acid loading. Persisting acidaemia may have contributed to higher plasma potassium in patients with CKD by decreasing sodium–hydrogen exchange and sodium–potassium ATPase activity in cells [4]. Furthermore, cumulative potassium excretion was lower in patients with CKD, which may also have contributed to the increase in plasma potassium. Aldosterone was not a limiting factor for potassium secretion, as this increased in patients with CKD. It is well characterized that metabolic acidosis induces natriuresis initially [5–7], and we also observed this. In patients with CKD, sodium excretion was lower, although—similar to ammonium—per-nephron sodium excretion was likely higher. Patients with CKD developed higher plasma aldosterone levels after acid loading, with higher plasma potassium or acidosis as potential drivers [8]. Another interesting observation was that the acid load acutely increased the excretion of creatinine, albumin and low molecular weight proteins. This could be caused by glomerular hyperfiltration or an effect on the proximal tubule (decreased protein reabsorption, increased creatinine secretion). We favour hyperfiltration as an explanation because this was previously observed after ammonium chloride loading in children [7] and rats [9] and because a previous micropuncture study did not find evidence for inhibition of proximal tubular reabsorption [6]. However, measurement of glomerular filtration rate would have been necessary to differentiate between the two possibilities.
Our data add potential explanations why metabolic acidosis in patients with CKD may contribute to adverse outcomes [2]. First, longer-lasting acidaemia causes a greater increase in plasma potassium and will therefore increase susceptibility to hyperkalaemia and its complications [10]. Second, the lower pH and higher plasma potassium increase plasma aldosterone, which may promote kidney fibrosis [11]. Third, acidosis increases proteinuria, which has been identified as a risk factor for progression of CKD [12]. Although this change also occurred in healthy volunteers, the acid load exposes patients with CKD to a greater degree of proteinuria, which may contribute to further kidney injury. Moreover, the acid load did cause a greater increase in urinary renin in patients than in healthy subjects, indicating differences between individual proteins [13]. The limitation of this study is that we did not include measurements of glomerular haemodynamics. Future studies are needed to evaluate if the identified differences in the acute response to acid loading also play a role in the long-term outcomes of CKD. In summary, we show that CKD alters the response to an acute acid load and we propose that this altered response may explain some of the associations between metabolic acidosis and outcomes in CKD.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all patients and healthy volunteers who participated in this study. We thank Drs Burling and Barker (Core Biochemical Assay Laboratory, Cambridge, UK) for their help with the retinol-binding protein measurements.
FUNDING
This work was supported by the Dutch Kidney Foundation (KSP 14OKG19).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Raphael KL. Metabolic acidosis in CKD: core curriculum 2019. Am J Kidney Dis 2019; 74: 263–275 [DOI] [PubMed] [Google Scholar]
- 2. Wesson DE, Buysse JM, Bushinsky DA.. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol 2020; 31: 469–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wrong O, Davies HE.. The excretion of acid in renal disease. Q J Med 1959; 28: 259–313 [PubMed] [Google Scholar]
- 4. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10: 1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faroqui S, Sheriff S, Amlal H.. Metabolic acidosis has dual effects on sodium handling by rat kidney. Am J Physiol Renal Physiol 2006; 291: F322–F331 [DOI] [PubMed] [Google Scholar]
- 6. Dubb J, Goldberg M, Agus ZS.. Tubular effects of acute metabolic acidosis in the rat. J Lab Clin Med 1977; 90: 318–323 [PubMed] [Google Scholar]
- 7. Gyorke ZS, Sulyok E, Guignard JP.. Ammonium chloride metabolic acidosis and the activity of renin-angiotensin-aldosterone system in children. Eur J Pediatr 1991; 150: 547–549 [DOI] [PubMed] [Google Scholar]
- 8. Wagner CA. Effect of mineralocorticoids on acid-base balance. Nephron Physiol 2014; 128: 26–34 [DOI] [PubMed] [Google Scholar]
- 9. Tammaro G, Zacchia M, Zona E. et al. Acute and chronic effects of metabolic acidosis on renal function and structure. J Nephrol 2018; 31: 551–559 [DOI] [PubMed] [Google Scholar]
- 10. Kovesdy CP, Matsushita K, Sang Y. et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 2018; 39: 1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remuzzi G, Cattaneo D, Perico N.. The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol 2008; 19: 1459–1462 [DOI] [PubMed] [Google Scholar]
- 12. Abbate M, Zoja C, Remuzzi G.. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17: 2974–2984 [DOI] [PubMed] [Google Scholar]
- 13. Roksnoer LC, Heijnen BF, Nakano D. et al. On the origin of urinary renin: a translational approach. Hypertension 2016; 67: 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.