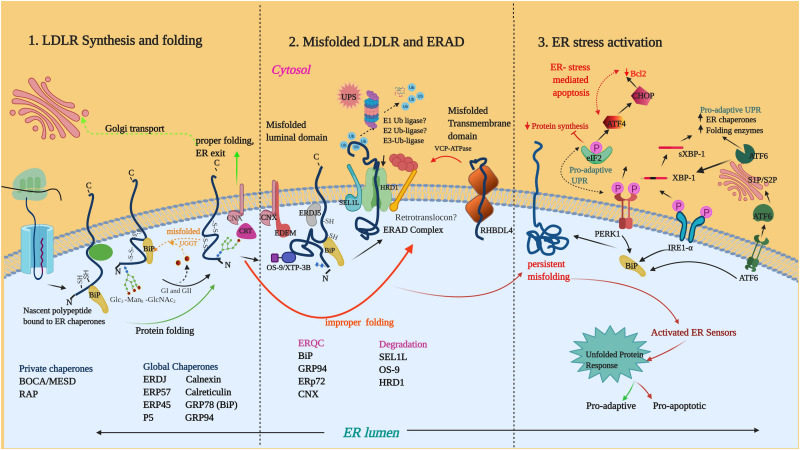
FIGURE 2.
LDLR folding, misfolding and activation of UPR: (1) The nascent LDLR is co-translationally inserted into the ER membrane and the LDLR ectodomain undergoes folding in the ER lumen with the assistance of several global and private chaperones as listed in the figure. (2) Misfolded proteins such as Class II mutants engage in prolonged interaction with the chaperone system. BiP, GRP94, ERP72 are ERQC factors implicated in LDLR retention. Terminally misfolded proteins are extracted from the chaperone system and delivered to membrane-embedded ERAD complex for degradation by the ubiquitin-proteasome system. So far, the components known to be involved in LDLR-ERAD are OS9, SEL1L and HRD1. RHBDL4 is a metalloprotease involved in the ERQC of ERAD-M candidates of LDLR. (3) Accumulation of misfolded LDLR induces ER stress and activates the UPR proteins IRE1, PERK, and ATF6. Phosphorylation of eIF2α by PERK leads to the attenuation of protein translation. Activated IRE1α induces splicing of the long XBP1 mRNA to form XBP1s mRNA which encodes XBP1s protein. Activated ATF6 is cleaved in the Golgi to form the active ATF6 N-terminal fragment. XBP1s and ATF6 are transcription factors that target the transcriptional induction of UPR target genes. Unresolved ER-stress turn-on proapoptotic pathways through the PERK-arm of the UPR. Illustration created with Biorender.com.