Abstract
Introduction
Upper urinary tract urothelial carcinomas are very rare tumours with different biological behaviours. The Epstein–Barr virus, which is the first known oncogenic virus, is being investigated for various malignant tumours. It is known that this virus is associated with nasopharyngeal carcinoma, as well as multiple haematological malignancies, head and neck and gastric cancers. We aimed to determine the presence of the Epstein–Barr virus in upper urinary tract urothelial carcinomas using chromogenic in situ hybridisation (CISH).
Materials and methods
A total of 44 upper urinary tract urothelial carcinomas from two different centres were included. Demographic data and survival rates were obtained from hospital records. One demonstrative paraffin block from each case was stained using Epstein–Barr encoded RNA (EBER) with an automated CISH procedure. The positivity of EBER was statistically analysed for prognostic factors.
Results
Among all patients, 38 were male and 6 were female. The mean age of the patients was 65.93 years. At the time of the study, 15 patients had died and 29 were alive. EBER-CISH positivity was found in 13 patients. Four showed strong EBER-CISH expression and nine showed weak expression. EBER-CISH positivity was not statistically related to any of the prognostic factors or to overall survival.
Discussion
Although EBER-CISH positivity showed no significant relation with prognostic factors, it was observed in one-third of all cases. Therefore, we think that the Epstein–Barr virus may have a role in the pathogenesis of upper urinary tract urothelial carcinomas. This finding needs to be supported by larger studies.
Keywords: Upper urinary tract urothelial carcinoma, Epstein–Barr virus, Chromogenic in situ hybridisation, Surgical pathology, Pathology of tumours
Introduction
Urothelial carcinoma can be seen anywhere along the urinary tract. The most common localisation is the bladder.1 However, it may arise rarely in the renal pelvis. As this localisation is very rare, the exact behaviour of carcinoma localised in the upper urinary tract remains a mystery.2
The Epstein–Barr virus, which is a member of the human herpesvirus family, is the first known oncogenic virus and has been found associated with various tumours.3 The most well-documented association between tumour types and the Epstein–Barr virus are the nasopharyngeal carcinoma, Hodgkin lymphoma, Burkitt lymphoma and diffuse large B-cell lymphoma of the elderly.4 However, many different tumours have been investigated to determine whether the Epstein–Barr virus plays a role in their pathogenesis.
Many different techniques can be used to detect the Epstein–Barr virus, such as polymerase chain reaction (PCR), real-time PCR, immunohistochemistry and in situ hybridisation. Generally, the viral products (encoded RNAs and proteins) were the targets for detection, in addition to the viral genomic DNA.5–7 The sensitivity and specificity vary depending on the technique used for detection.5
Epstein–Barr virus-encoded small RNAs (EBERs) are small noncoding, non-polyadenylated RNAs that serve as infection markers for the Epstein–Barr virus and are present in latently infected cells.8 Epstein–Barr virus nuclear antigens (EBNAs) regulate gene expression and latent membrane proteins, which is connected to the persistency of the virus, activating the signalling pathways and inhibiting human epithelial cell differentiation by corrupting the cellular response to differentiation signals.9–11
The upper urinary tract is a rare localisation for urothelial carcinoma.1 Despite its rarity, urothelial carcinomas are the most common malignant tumour type of the upper urinary tract. In this study, we aimed to detect Epstein–Barr virus positivity in tumour cells of the upper urinary tract urothelial carcinoma by using EBER chromogenic in situ hybridisation (CISH). To the best of our knowledge, this is the first study to focus on urothelial carcinoma of this localisation in the literature.
Materials and methods
Forty-four patients from two centres who underwent surgical excision procedures (partial nephrectomy/ureterectomy/nephroureterectomy/nephrocystoprostatectomy) and were diagnosed as having upper urinary tract urothelial carcinoma were included in this study. Patients who were out of follow-up or treated by another centre were excluded. Patients whose paraffin blocks could not be reached because of being examined by another centre were also excluded. Written informed consent was obtained from all patients included in the study.
Demographic data (eg age, sex, location) and other prognostic features (tumour size, stage, lymphovascular invasion, perineural invasion, surgical margins) were obtained from pathology reports and hospital records. The current status (dead/alive) of the patients was obtained from the medical records.
The haematoxylin and eosin-stained slides were revised by three pathologists and the features of the tumours were noted. Among many prognostic factors, the size, location (pelvis and/or ureter), stage and grade of the tumour, presence of lymphovascular/perineural invasion, necrosis, synchronous or metachronous bladder cancer, recurrence and distant metastasis, and status of the surgical margins and lymph nodes were noted. In addition, the differentiation of the tumours (squamous/glandular/sarcomatoid/none) was taken into consideration. The total time of follow-up (months), disease-free survival time for recurrent patients (months) and the total life span for dead patients (months) were also noted for prognostic analysis.
From the paraffin blocks of the patients, a representative block was chosen and four-micrometre paraffin sections were prepared from these formalin-fixed tissues from surgical specimens for CISH automatic staining using an EBER probe (Leica Biosystems) with Leica BOND-MAX. Nasopharyngeal carcinoma tissue was used as a positive control. We were only able to perform staining procedure to upper urinary tumours and we could not stain meta/synchronous tumours due to limited EBER-CISH staining kits. All tumoural haematoxylin and eosin sections and CISH-stained slides were then assessed for additional features and EBER positivity by three pathologists. Even one cell expressing EBER-CISH was accepted as positive staining.
The correlation of overall survival with the presence of lymph node metastasis, necrosis, lymphovascular invasion, perineural invasion and EBER-CISH positivity was investigated using Kaplan–Meier and log-rank analysis. A p-value less than 0.05 was considered as the level of statistical significance. Statistical analyses were performed using the Statistical Package for the Social Sciences Version 24 statistical software. This study was approved by the Ethics Committee of Human Investigations of Muğla Sıtkı Koçman University (82/18).
Results
There were 44 patients included in the study, with a mean age of 65.93 ± 8.87 years. Thirty-eight (86.4%) patients were male and six (13.6%) were female. The characteristic features of the tumours are given in Table 1.
Table 1.
General features of the tumours
| Feature | Cases | |
| (n) | (%) | |
| Stage: | ||
| pTa | 8 | 18.2 |
| pT1 | 6 | 13.6 |
| pT2 | 2 | 4.5 |
| pT3 | 20 | 45.5 |
| pT4 | 8 | 18.2 |
| Grade: | ||
| Low | 10 | 22.7 |
| High | 34 | 77.3 |
| Side: | ||
| Right kidney | 22 | 50 |
| Left kidney | 22 | 50 |
| Localisation: | ||
| Renal pelvis | 21 | 47.7 |
| Ureter | 3 | 6.8 |
| Renal pelvis and ureter | 20 | 45.5 |
| Additional differentiation: | ||
| None | 27 | 61.4 |
| Squamous | 14 | 31.8 |
| Sarcomatoid | 2 | 4.5 |
| Glandular | 1 | 2.3 |
| Synchronous/metachronous: | ||
| No | 24 | 54.5 |
| Bladder urothelial carcinoma | 20 | 45.5 |
Among the general histopathological prognostic factors, lymph node metastasis was observed in 8 cases (18.2%), necrosis in 24 cases (54.5%), lymphovascular invasion in 19 cases (43.2%), and perineural invasion in 5 cases (11.4%).
Of the total, 15 (34.1%) patients were dead and 29 (65.9%) alive. Perineural invasion had a strong relation with overall survival (p = 0.055) and surgical margin positivity had a significant relation with factors that showed a relation with overall survival. These factors and others that were thought to be associated with overall survival are summarised in Table 2.
Table 2.
Histopathological factors related to overall survival
| Factor | Positive | Negative | p-value | ||
| (n) | (%) | (n) | (%) | ||
| Lymph node metastasis | 8 | 18.2 | 36 | 81.8 | 0.796 |
| Tumour necrosis | 24 | 54.5 | 20 | 45.5 | 0.522 |
| Lymphovascular invasion | 19 | 43.2 | 25 | 56.8 | 0.682 |
| Perineural invasion | 5 | 11.4 | 39 | 88.6 | 0.055 |
| Surgical margin positivity | 6 | 13.6 | 38 | 86.4 | 0.013 |
| Distant metastasis | 14 | 31.8 | 30 | 68.2 | 0.923 |
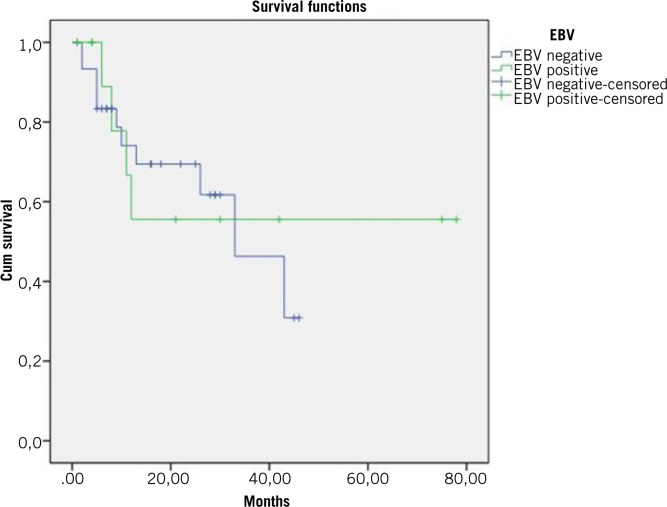
Among all patients, 13 (29.5%) showed EBER-CISH positivity. The positivity was mild in most of these patients (9/13 cases), but four patients had strong positivity. EBER-CISH positivity was not significantly related to overall survival (p = 0.845, Figures 1 and 2) or any of the prognostic factors mentioned above.
Figure 1.
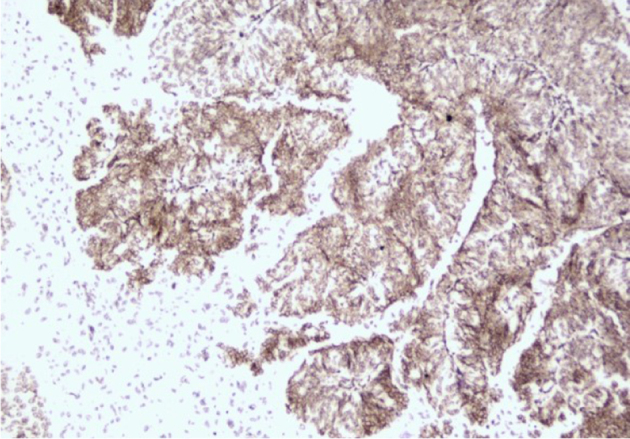
Strong positivity of EBER-CISH, X200, DAB
Figure 2.
The relation between EBER-CISH expression with overall survival, Kaplan–Meier analysis
The mean disease-free survival was 62 months (minimum 52 months, maximum 73 months). In our study group, seven patients had recurrent disease (11.6%): five men and two women. We found a statistically significant relation between sex and disease-free survival (p = 0.047). When we evaluated the pathological stage among the seven patients with recurrent disease, four were pT3, two were pT4 and one was pTa. There was no significant relation between disease-free survival and pathological stage (p = 0.741). However, recurrence was more common in patients with advanced disease. Two of seven recurrent cases were low-grade while five were high-grade tumours but there was no significant relationship between differentiation and disease-free survival (p = 0.688).
We also analysed the relationship between disease-free survival and EBER-CISH expression. Among the seven recurrent cases, only one showed mild EBER-CISH positivity and the relationship was not significant (p = 0.595).
One of the most important and significant findings is the relationship between recurrent disease and the presence of metachronous or synchronous tumour. Six of seven recurrent cases had meta/synchronous bladder tumour and this relationship was statistically significant (p = 0.008)
Discussion
The Epstein–Barr virus, a member of the human herpesvirus family, is a DNA virus that causes a transient infection known as infectious mononucleosis. Its importance lies in it remaining as a latent virus within the memory B cells after primary infection.12,13 Many techniques can be used to detect Epstein–Barr virus and sensitivity and specificity changes according to the technique. Epstein–Barr virus latent membrane protein 1 (LMP-1) antibodies reflect latent infections. PCR and DNA analysis target viral products, viral DNA, and show viral load.5–7 EBERs are small noncoding RNAs that indicate EBV infectious markers.10,11
Inflammation and cancer have been researched for many years.14–16 The latency of the Epstein–Barr virus is the major factor that causes chronic inflammation and oncogenic changes. Epstein–Barr virus-related malignancies are associated with viral proteins, which regulate proliferation, the immune system, immune response and cell apoptosis.11 The virus has been detected in a variety of lymphoid, epithelial and mesenchymal tumours, and even benign tumours such as leiomyomas.3,16
The Epstein–Barr virus has been shown as a cause for several types of epithelial malignancies, such as head and neck tumours, nasopharyngeal cancer, gastric carcinomas, in addition to B-lymphocytic malignancies such as Burkitt and Hodgkin lymphomas.17–22 The most common head and neck tumours associated with the Epstein–Barr virus are nasopharyngeal and squamous cell carcinomas. A meta-analysis showed that Epstein–Barr viral infections might be associated with an increased risk of oral squamous cell carcinoma.13
Epstein–Barr virus infection was found to be associated with chronic interstitial nephritis in the study of Shimakage et al.5 The same study suggested that there might be a link between chronic interstitial nephritis and renal cell carcinoma, which was supported by the study, reporting EBNA2 expression in kidney tubule cells in induced renal tumours.23
Among genitourinary cancers, Epstein–Barr virus expression has mostly been analysed in renal tumours. In one study, the virus was expressed in cells with renal cell carcinomas, but expression was rare in normal kidney or other renal diseases.20 It was stronger in papillary and clear-cell renal cell carcinoma than in chromophobe cell renal cell carcinoma. It was expressed more strongly in high-grade renal cell carcinoma than in low-grade renal cell carcinoma.20 Kwang et al found that Epstein–Barr viral infection was related to sarcomatoid renal cell carcinoma tissues.24 The association with tumours with sarcomatoid differentiation remains unclear because of the small number of studies.22
The relationship between upper urinary tract urothelial carcinoma and the Epstein–Barr virus in the development of urothelial carcinomas is unclear. The study of Chuang et al is the first example of reports focusing on Epstein–Barr virus-infected B-cell lines associated with urothelial carcinoma.21 However, they stated that it would be important to show whether the Epstein–Barr virus DNA variant and the mutant LMP-1 detected in infected B cells could also be detected in the urothelial carcinoma tumour cells. That was the main target of our study. We tried to demonstrate the presence of EBER positivity in renal pelvis urothelial carcinoma cells and detected EBER-CISH positivity in 29.5% of our cases.
Most recently, Epstein–Barr virus DNA was determined in 66 bladder urothelial carcinoma specimens using real-time PCR. In non-muscle invasive urothelial tumours, poor differentiation was found to be correlated with the high load of the Epstein–Barr virus genome.25 In contrast to this finding, we found no significant correlation between the stage and Epstein–Barr virus CISH positivity (p = 0.89) and most of the cases showing Epstein–Barr virus CISH positivity were pT3 (n = 7/13). However, this could be related to the rarity of upper urinary tract urothelial carcinomas when compared with bladder urothelial carcinomas.
Conclusions
Our results may draw attention to the possible role of an Epstein–Barr virus variant in upper urinary tract urothelial carcinoma. However, confirming the exact aetiological role of Epstein–Barr virus in upper urinary tract urothelial carcinomas should be supported by larger studies with larger numbers of cases.
References
- 1.Grignon DC, Lloreta J, Al-Ahmedie H et al. . Infiltrating urothelial carcinoma, chapter 2: Tumors of the urinary tract In: Moch H, Humphrey PA, Ulbright TM, Reuter VE (eds), WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed Lyon: IARC; 2016, pp77–98. [Google Scholar]
- 2.Ekmekçi S, Küçük Ü, Dere Y, et al. 8-armed octopus: evaluation of clinicopathologic prognostic factors of urothelial carcinoma of the upper urinary system. Turk J Med Sci 2019; : 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PG, Young LS. Epstein–Barr virus infection: basis of malignancy and potential for therapy. Expert Rev Mol Med 2001; : 1–20. [DOI] [PubMed] [Google Scholar]
- 4.Rickinson AB, Kieff E. Epstein-Barr virus In: Knipe DM, Howley PM (eds), Fields Virology. 5th ed Philadelphia, PA: Lippincott-Raven; 2007, pp2603–2654. [Google Scholar]
- 5.Shimakage M, Horii K, Tempaku A et al. . Association of Epstein-Barr virus with oral cancers. Hum Pathol 2002; : 608–614. [DOI] [PubMed] [Google Scholar]
- 6.Jiang R, Gu X, Moore-Medlin TN et al. . Oral dysplasia and squamous cell carcinoma: correlation between increased expression of CD21, Epstein–Barr virus and CK19. Oral Oncol 2012; : 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Noguchi Y, Hoshino M et al. . Detection of Epstein–Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumour Biol 2016; : 3389–3404. [DOI] [PubMed] [Google Scholar]
- 8.Rosa MD, Gottlieb E, Lerner MR, Steitz JA. Striking similarities are exhibited by two small Epstein–Barr virus-coded ribonucleic acids and adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol 1981; : 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson CW, Rickinson AB, Young LS. Epstein–Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature 1990; : 777–780. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Metgud R. Evidences suggesting involvement of viruses in oral squamous cell carcinoma. Patholog Res Int 2013; : 642496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 2014; : 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salehipoor M, Khezri A, Behzad-Behbahani A. Role of viruses in renal cell carcinoma. Saudi J Kidney Dis Transpl 2012; : 53–57. [PubMed] [Google Scholar]
- 13.She Y, Nong X, Zhang M, Wang M. Epstein–Barr virus infection and oral squamous cell carcinoma risk: a meta-analysis. PLoS ONE 2017; : e0186860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkwill F, Manntovani A. Inflammation and cancer: back to Virchow?. Lancet 2001; : 539–545. [DOI] [PubMed] [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; : 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan R, Freeman JA, Creager AJ. Epstein–Barr virus induced renal leiomyoma. J Urol 1999; : 212. [PubMed] [Google Scholar]
- 17.Prabhu SR, Wilson DF. Evidence of Epstein–Barr virus association with head and neck cancers: a review. J Can Dent Assoc 2016; : g2. [PubMed] [Google Scholar]
- 18.Thompson MP, Kurzrock R. Epstein–Barr virus and cancer. Clin Cancer Res 2004; : 803–821. [DOI] [PubMed] [Google Scholar]
- 19.Grywalska E, Rolinski J. Epstein–Barr virus-associated lymphomas. Semin Oncol 2015; : 291–303. [DOI] [PubMed] [Google Scholar]
- 20.Shimakage M, Kawahara K, Harada S et al. . Expression of Epstein–Barr virus in renal cell carcinoma. Oncol Rep 2007; ; 41–46. [PubMed] [Google Scholar]
- 21.Chuang CK, Chuang KL, Hsieh CH et al. . Epstein–Barr virus-infected cell line TCC36B derived from B lymphocytes infiltrating renal pelvis urothelial carcinoma. Anticancer Res 2010; : 3473–3478. [PubMed] [Google Scholar]
- 22.Colegate-Sonate TJ, Rao AR, Callaghan PS. Epstein–Barr virus infection in sarcomatoid renal cell carcinoma tissues. BJU Int 2006; : 197–199. [DOI] [PubMed] [Google Scholar]
- 23.Novick AC, Campbell SC: Renal tumor Chapter 75 Vol. 4 In: Walsh PC, Retik AB, Vaughan ED et al.. (eds) Campbell’s Urology. 8th ed Philadelphia, PA: WB Saunders; 2002, pp2672–2731. [Google Scholar]
- 24.Kim KH, Han EM, Lee ES et al. . Epstein–Barr virus infection in sarcomatoid renal cell carcinoma tissues. BJU Int 2005; : 547–552. [DOI] [PubMed] [Google Scholar]
- 25.Chuang KL, Pang ST, Liao SK et al. . Epstein–Barr virus DNA load in tumour tissues correlates with poor differentiation status in non-muscle invasive urothelial carcinomas. BJU Int 2010; : 150–154. [DOI] [PubMed] [Google Scholar]