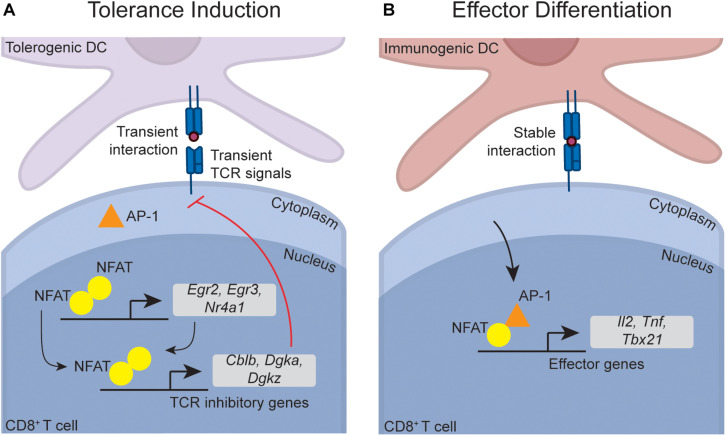
FIGURE 2.
Putative transcriptional pathways that program CD8+ T cell tolerance. Although not directly demonstrated within CD8+ T cells, data suggest that the NFAT-dependent transcriptional programs that induce CD4+ T cell anergy also operate during CD8+ T cell tolerance. During tolerance induction (A), transient naïve CD8+ T cell interactions with DCs (which can occur due to PD-1 and CTLA-4 signaling) lead to an accumulation of NFAT in the nucleus in the absence of AP-1. This is because AP-1 is rapidly exported from the nucleus upon antigen withdrawal, while NFAT is slowly exported, meaning that transient interactions lead to preferential nuclear NFAT accumulation. In the absence of AP-1, NFAT forms low affinity homodimers that induce transcription factors such as Egr2, Egr3, and Nr4a1 (also called Nur77). These transcription factors, in combination with NFAT, induce a range of factors that directly inhibit TCR signaling (such as Cblb, Dgka, and Dgkz), thereby enforcing anergy. During effector differentiation (B), stable DC-T cell interactions enable sustained TCR signaling, leading to accumulation of both AP-1 and NFAT within the nucleus. NFAT-AP-1 complexes are higher affinity than NFAT homodimers, meaning that in the presence of AP-1, NFAT-AP-1 complexes preferentially form. When complexed with AP-1, NFAT preferentially induces effector genes such as the cytokines Il2 and Tnf, and Tbx21 (encoding TBET), which in turn induces Ifng.