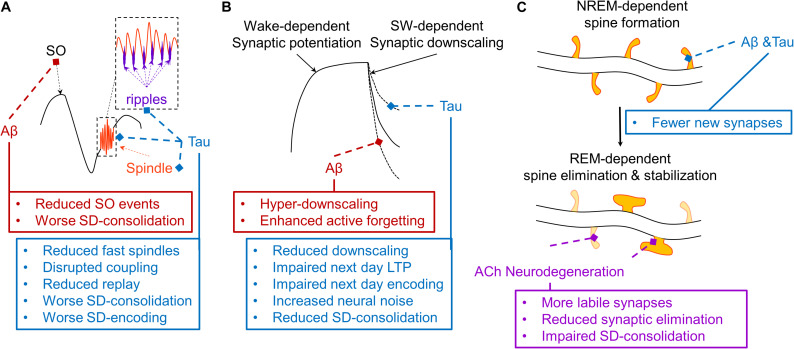
FIGURE 3.
Schematic depiction of hypothesized effects of AD pathophysiology on mechanisms of three models of sleep-dependent memory consolidation. (A) A theoretical depiction of the mechanisms supporting active systems consolidation (Steriade, 2006; Diekelmann and Born, 2010; Watson and Buzsaki, 2015). The thalamic sleep spindle (orange) is phase-locked to the depolarizing upstate of the slow oscillation (SO, black), and the hippocampal ripple is phase-locked to the troughs of the sleep spindle (purple), supporting coordinated replay of memory traces which triggers the cortical plasticity necessary for systems consolidation. Aβ (red) selectively disrupts SO expression, impairing sleep-dependent (SD) memory consolidation. Tau (blue) disrupts fast sleep spindle and ripple expression and their coupling with the SO, disrupting memory replay and related memory consolidation and subsequent encoding (Walker, 2009; Watson and Buzsaki, 2015; Mander et al., 2017a). (B) A theoretical depiction of the sleep homeostasis hypothesis (SHY), which posits that slow waves (SW) facilitate global synaptic downscaling in response to widespread synaptic potentiation following waking experience (Tononi and Cirelli, 2014). Aβ increases delta waves, which may increase the magnitude of synaptic downscaling, potentially overriding local signals protecting relevant synapses from long-term depression (LTD), ultimately exaggerating an active forgetting process. Tau reduces overall slow wave activity, which may weaken the process of synaptic downscaling, resulting in increased neural noise and impaired sleep-dependent memory consolidation. Another consequence could be a brain that remains more over-potentiated, thus limiting the capacity to induce long-term potentiation (LTP), resulting in impaired next day learning. (C) A theoretical depiction of a NREM-REM two-stage model of synaptic plasticity supporting sleep-dependent memory (Yang et al., 2014; Miyawaki and Diba, 2016; Li et al., 2017; Mizuseki and Miyawaki, 2017). In this model, NREM sleep facilitates the formation of a small number of dendritic spines on dendritic branches of neurons triggered by learning experiences during prior wakefulness. This is followed by a REM sleep-dependent process that eliminates most of these new labile dendritic spines, but stabilizes some that most support successful memory formation. Through their disruption of memory-relevant NREM sleep oscillations, both Aβ and tau could reduce the number of new dendritic spines formed following a learning experience. Through cholinergic degeneration (purple), and the resulting impairments in REM sleep physiology, this could be further compounded by impaired REM sleep-dependent elimination and stabilization of relevant dendritic spines. This could result in greater neural noise, faster memory trace decay, and a reduction in resistance to interference, exacerbating forgetting.