Abstract
Objectives
To compare the sedative effects of dexmedetomidine alfentanil versus ketamine-alfentanil in patients undergoing closed reduction of nasal fractures on the basis of intraoperative hemodynamic changes, satisfaction of patients and surgeons, and the adverse effects.
Methods
Sixty patients with ASA class 1 or 2 were randomized to either of two groups, a dexmedetomidine alfentanil group (DA group; n = 30) or a ketamine-alfentanil group (KA group; n = 30). Hemodynamic parameters, oxygenation status, adverse events, the satisfaction of patients and surgeons, and postoperative pain scores by visual analog scale (VAS) were recorded at specific time intervals during the trial.
Results
Systolic blood pressure was significantly lower in the DA group than in the KA group from T1 min to T15 min. The duration of the recovery ward stay was longer in the DA group; however, two groups were similar in terms of total anesthesia time and awakening time. Likewise, two groups were similar in terms of the patient and surgeon’s satisfaction, pain scores, and the occurrence of adverse effects.
Conclusions
Both sedation methods were safely performed, and dexmedetomidine-alfentanil is as effective as ketamine-alfentanil in patients undergoing short-term operations such as nasal fracture corrections.
Keywords: Dexmedetomidine, Ketamine, Alfentanil, Nasal Fracture
1. Background
Nasal bone fractures are the most common type of facial fractures (1). One treatment for this type of fracture is a closed reduction that can be done under local or general anesthesia (2). General anesthesia provides better patient satisfaction with anesthesia, appearance, and function of the nose and reduces the corrective surgeries such as septoplasty, septorhinoplasty, and rhinoplasty (3). However, it may not be an option because of medical risk factors, emergent need for intervention, or operating room availability. Intravenous sedation may be the most controllable way of determining the exact level of ideal sedation individually needed for short-term operations such as nasal manipulations (4, 5).
Dexmedetomidine is a highly selective α2 agonist that reduces peripheral norepinephrine secretion by stimulating inhibitory prejunctional α2 adrenoreceptors. The half-life of dexmedetomidine is 2.3 hours and its distribution half-life is less than 5 minutes; so, its clinical effects are very rapid (6-8). Also, it assists hemodynamic stability by its sympatholytic action, which has been used as a safe adjuvant during anesthesia in many clinical applications (9-12). Dexmedetomidine has been used as a sedative and hypnotic in procedures that do not need tracheal intubation. Since dexmedetomidine has no negative effect on respiratory rate or depth than other sedatives, it has been proven that it is usable in airway-related procedures (13, 14). However, ketamine is a dissociative anesthetic that plays a significant role in analgesia and sedation for outpatient surgeries. It has not only sedative-hypnotic properties resulting in a light dissociative sleep but also has potent analgesic properties (15, 16).
Closed reduction of nasal fractures is a short-term operation that needs awakening closely after anesthetic induction. Therefore, the opioids administered during the operation should be short-acting to provide a rapid awakening from anesthesia. Alfentanil is a potent, rapid, and short-acting analog of fentanyl, which appears to have some significant advantages during outpatient surgeries (17).
2. Objectives
The aim of this study was to compare the sedative effects of dexmedetomidine alfentanil versus ketamine-alfentanil in patients undergoing closed reduction of nasal fractures.
3. Methods
This randomized, double-blind clinical trial (code: IRCT20191127045530N1) was approved by the Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences (code: IR.AJUMS.REC.1398.624) and was conducted in Imam Khomeini Hospital, Ahvaz, Iran from Jan to February 2020. The advantages and disadvantages of sedation were explained to the patients before the operation.
The patients were randomly divided into two groups, including the dexmedetomidine alfentanil group (DA group; n = 30) and the ketamine-alfentanil group (KA group; n = 30). Patients with ASA class I or II, aged 15 to 40, were scheduled to reduce nasal bone fracture. Patients under 15 and over 40 years, patients with a history of analgesic consumption within 24 hours before the surgery, uncontrollable hypertension or heart diseases, coagulopathy, a history of convulsion, and neurologic disorders were excluded from the study.
Blood pressure, heart rate, and peripheral oxygen saturation (SpO2) were continuously monitored in patients entered the operating room every 5 min. At least 5 min prior to the operation, all patients received supplementary 100% oxygen at a rate of 5 - 6 lit/min through a face mask.
Both groups received 0.05 mg/kg midazolam (Chemidarou, Iran) and 0.01 mg/kg atropine (Caspian, Iran) intravenously before anesthetic induction. Then, in the dexmedetomidine-alfentanil (DA) group, dexmedetomidine (Exir, Iran) was administered at a rate of 1 µg/kg over 10 min in combination with 8 µg/kg alfentanil (Darou Pakhsh, Iran) to give a sedation level of 5 - 6 according to Ramsay sedation scale (Table 1). In the ketamine-alfentanil (KA) group, sedation was induced with bolus injection of ketamine 1mg/kg (Rotexmedica, Germany), 8 µg/kg alfentanil to keep the same level of sedation. Discomfort and agitation were considered indicators of inadequate sedation and were treated with i.v. Propofol 20 mg (Fresenius Kabi, Austria).
Table 1. Ramsay Sedation Scale (18).
| Score | Details |
|---|---|
| 1 | Patient is anxious or agitated |
| 2 | The patient is cooperative, oriented, and tranquil |
| 3 | Patient responds to commands only |
| 4 | Patient exhibits brisk response to light glabellar tap or loud auditory stimulus |
| 5 | Patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus |
| 6 | Patient exhibits no response |
In both groups, changes in sBP, dBP, MAP, HR, and SpO2 were recorded by staff members who were blinded to the patients’ group allocation at specific time intervals. Anesthesia time, awakening time (based on Ramsay sedation score: 3), and the recovery ward stay duration (based on Modified Alderete score: 9) were recorded. After surgery, satisfaction of the patients and surgeon was measured (7-point Likert-like verbal rating scale), and the pain was evaluated using the visual analogue scale (VAS, 0: no pain-10 cm: worst pain) at specific intervals until six hours.
All variables were evaluated by an investigator who was blinded to the patient group. SPSS 26 software was used for statistical analysis. All values were expressed as mean (SD), median (range), or the number of patients (%). Student t-test was used to compare continuous variables, and repeated measures analysis of variance (ANOVA) was used to analyze hemodynamic changes over time. A chi-square test was used to analyze categorical variables. P values of less than 0.05 were considered statistically significant.
4. Results
There was no statistically significant difference between the groups in age, sex, and weight of the patients (Table 2). Two groups were similar in terms of the patient and surgeon’s satisfaction and also no significant differences in the occurrence of nausea, agitation, and lethargy (Table 3). The postoperative pain was assessed using the Visual Analogue scale (VAS) before induction, in the recovery ward, 2, and 6 hours postoperatively. The results showed that there was no significant difference between the two groups (Table 4). The duration of the recovery ward stay was longer in the DA group (36.83 (8.03) min vs. 30.00 (7.76) min, P < 0.001); however, the two groups were similar in terms of total anesthesia time and awakening time (Table 5).
Table 2. Demographic Characteristics of the Patientsa.
| DA Group (N = 30) | KA Group (N = 30) | P Value | |
|---|---|---|---|
| Age, y | 26.27 ± 6.90 | 27.07 ± 7.40 | 0.66 |
| Male/female | 25/5 | 22/8 | 0.53 |
| Weight, kg | 67.77 ± 13.55 | 70.63 ± 16.94 | 0.47 |
| ASA | 0.70 | ||
| 1 | 25 (0.83) | 27 (0.9) | |
| 2 | 5 (0.16) | 3 (0.1) |
Abbreviations: DA, dexmedetomidine-alfentanil; KA, ketamine-alfentanil.
aValues are expressed as mean ± SD or No. (%).
Table 3. Complications and Satisfaction of the Patients and Surgeon After the Operationa, b.
| DA Group (N = 30) | KA Group (N = 30) | P Value | |
|---|---|---|---|
| Nausea-vomiting | 5 (16.66) | 6 (20) | 0.73 |
| Agitation | 0 (0) | 2 (6.66) | 0.49 |
| Lethargy | 9 (30) | 2 (6.66) | 0.04c |
| Satisfaction-patient | 7 (3 - 7) | 7 (4 - 7) | 0.18 |
| Satisfaction-surgeon | 7 (4 - 7) | 7 (3 - 7) | 0.46 |
Abbreviations: DA, dexmedetomidine-alfentanil; KA, ketamine-alfentanil.
aValues are expressed as median (range) or No. (%).
bSatisfaction: 1, extremely dissatisfied; 2, dissatisfied; 3, somewhat dissatisfied; 4, undecided; 5, somewhat satisfied; 6, satisfied; 7, extremely satisfied.
cP value < 0.05.
Table 4. Visual Analogue Scale Scores Before and Postoperationa.
| VAS | DA Group (N = 30) | KA Group (N = 30) | P Value |
|---|---|---|---|
| Before induction | 0.47 ± 1.1 | 0.40 ± 0.8 | 0.79 |
| In the recovery ward | 2.00 ± 1.7 | 2.33 ± 2.1 | 0.50 |
| 2 hours postoperatively | 2.27 ± 1.7 | 2.47 ± 1.8 | 0.67 |
| 6 hours postoperatively | 1.13 ± 1.5 | 1.47 ± 1.6 | 0.42 |
Abbreviations: DA, dexmedetomidine-alfentanil; KA, ketamine-alfentanil; VAS, Visual Analogue scale.
aValues are expressed as mean ± SD.
Table 5. Operation-Related Dataa.
| DA Group (N = 30) | KA Group (N = 30) | P Value | |
|---|---|---|---|
| Anesthesia time, min | 21.33 ± 6.14 | 18.50 ± 5.74 | 0.07 |
| Awakening time (Ramsay sedation score: 3), min | 8.17 ± 2.91 | 9.90 ± 4.33 | 0.07 |
| The recovery ward stay duration (Modified Alderete score: 9), min | 36.83 ± 8.03 | 30.00 ± 7.76 | 0.001* |
Abbreviations: DA, dexmedetomidine-alfentanil; KA, ketamine-alfentanil.
aValues are expressed as mean ± SD.
bP value < 0.05.
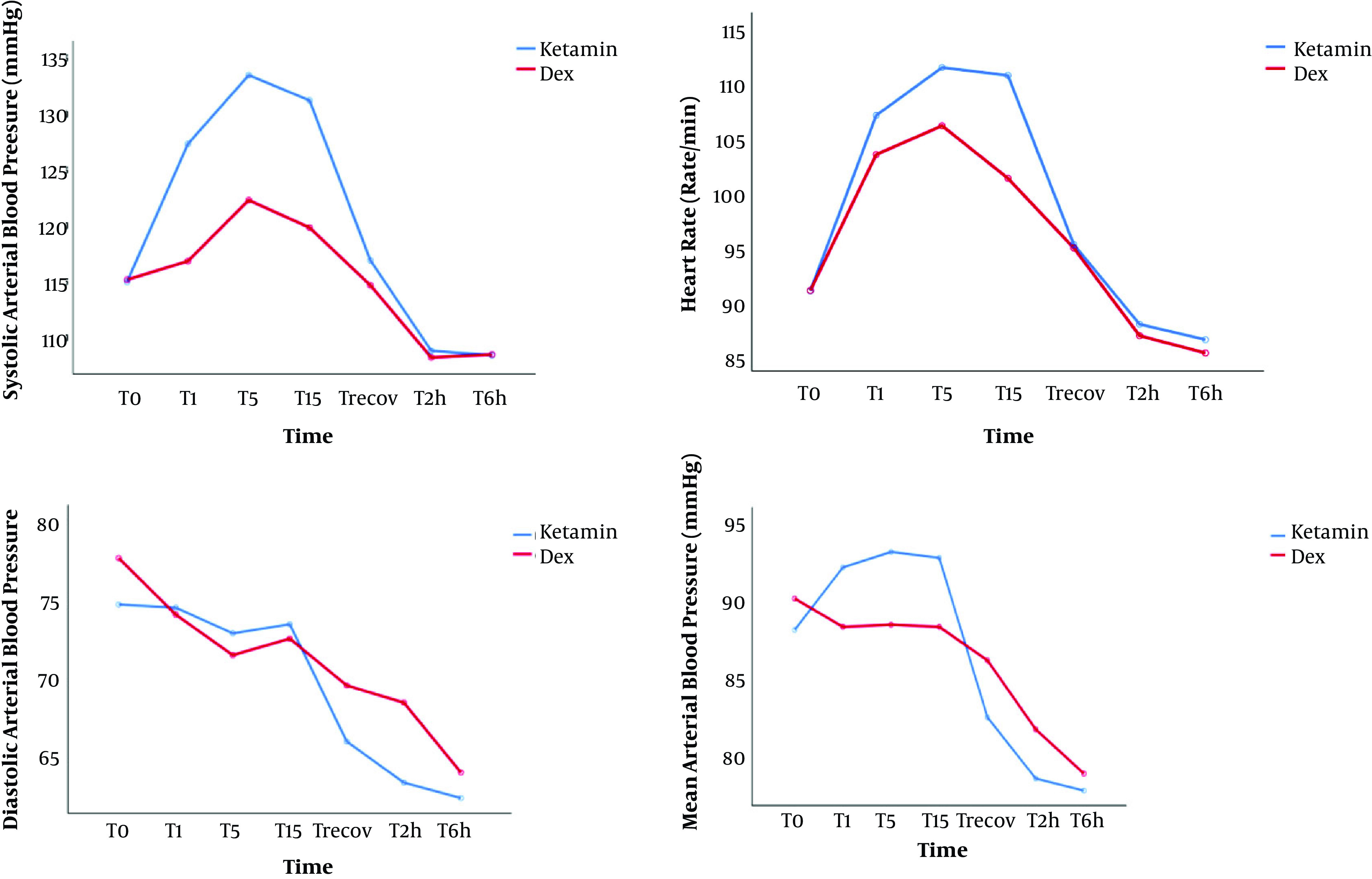
Regarding intraoperative hemodynamic changes, the DA group had a lower systolic BP, MAP, and HR compared to the KA group, but only systolic blood pressure changes from T1 min to T15 min were significantly different. (P < 0.01) (Figure 1).
Figure 1. Changes in hemodynamic variables. Data are shown as mean. DA: dexmedetomidine- alfentanil, KA: ketamine-alfentanil. T0: baseline, T1, T5, and T15: 1, 5, and 15 min after drug infusion in both groups, Trecov: at the recovery ward, T2h, and T6h: 2 and 6 hours after infusion.
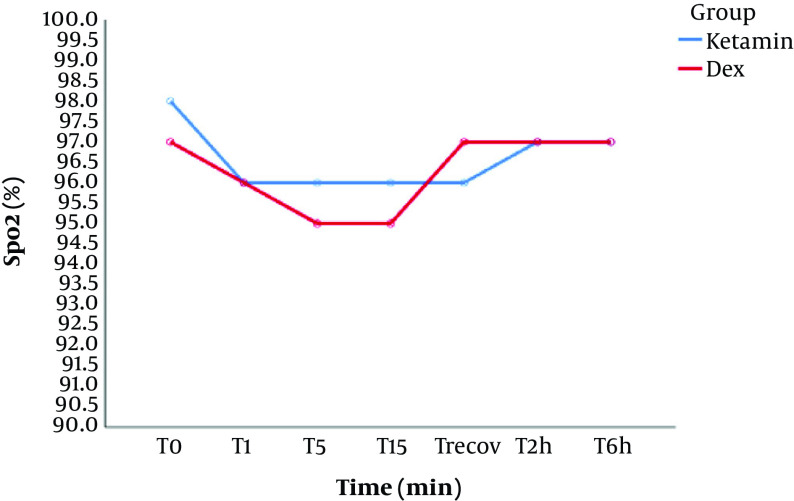
No episodes of desaturation (SpO2 < 90%) were noted during this study and there was no significant difference in SpO2 between both groups. (Figure 2)
Figure 2. Changes in peripheral oxygen saturation (SpO2). Data are shown as mean DA: dexmedetomidine- alfentanil, KA: ketamine-alfentanil. T0: baseline, T1, T5, and T15: 1, 5 and 15 min after drug infusion in both groups, T recov: at the recovery ward, T2h, and T6h: 2 and 6 hours after infusion.
5. Discussion
Sympatholytic property of dexmedetomidine provided lower systolic blood pressure during T1 min to T15 min of operation. Our finding was in accordance with studies that found a significant reduction in hemodynamic status using dexmedetomidine (19, 20). Also, Jae-Wook et al. (21) demonstrated that dexmedetomidine has sympatholytic effects and it is used for sedative, anxiolytic, and analgesic purposes as an adjunct to several anesthetics. So, these complex properties of dexmedetomidine led patients to experience less pain during the postoperative period in their study.
Kim et al. (22) stated that pre-operative administration of dexmedetomidine in a closed reduction of nasal bone fractures reduces agitation severity and shortens the duration of agitation and also provided more stable maintenance of anesthesia with less movement during the operation.
Our results confirmed the findings of Kim et al. (23) who conducted a randomized controlled trial of 100 nasal surgeries (the dexmedetomidine group received dexmedetomidine infusion, while the control group received normal saline as the placebo). Intra-operative continuous dexmedetomidine infusion resulted in more stable hemodynamics during extubation and enhanced patient-reported global quality of recovery 24 h after surgery.
The results of this study showed that two groups were similar in terms of the patient and surgeon’s satisfaction and postoperative pain scores, and also, there were no significant differences in the occurrence of adverse effects. In a similar study, Lee et al. (24) found that monitored anesthesia care with dexmedetomidine resulted in comparable satisfaction in the patients, nurses, and surgeons compared to general anesthesia. The occurrence of complications and pain scores were also similar.
One of the most common problems in the recovery ward is delayed recovery and longer discharge time of patients receiving pre-operative or intra-operative dexmedetomidine infusion. (19, 21, 22) In our research, the duration of the recovery ward stay was significantly longer in the DA group than in the KA group. However, two groups were similar in terms of total anesthesia time and awakening time.
5.1. Conclusions
This study was conducted to compare dexmedetomidine-alfentanil versus ketamine-alfentanil for sedation of patients undergoing closed reduction of a nasal bone fracture. Both sedation methods were performed without the incidence of any serious side effects, and two groups were similar in terms of the patient and surgeon’s satisfaction, pain scores and occurrence of complications. Therefore, dexmedetomidine-alfentanil is considered to be a good choice for sedation during nasal fracture corrections.
Footnotes
Authors' Contribution: None declared by author.
Clinical Trial Registration Code: The clinical trial registration code was IRCT20191127045530N1.
Conflict of Interests: No conflict of interest was declared.
Ethical Approval: The ethical approval code was IR.AJUMS.REC.1398.624.
Funding/Support: Ahvaz Jundishapur University supported this study.
Contributor Information
Reza Akhondzadeh, Email: rezaakh@hotmail.com.
Alireza Olapour, Email: olapour486@yahoo.com.
Mahboobe Rashidi, Email: rashidi.mahbobe@yahoo.com.
Fahimeh Elyasinia, Email: elyasinia.fahimeh@gmail.com.
References
- 1.Rhee SC, Kim YK, Cha JH, Kang SR, Park HS. Septal fracture in simple nasal bone fracture. Plast Reconstr Surg. 2004;113(1):45–52. doi: 10.1097/01.PRS.0000096705.64545.69. [DOI] [PubMed] [Google Scholar]
- 2.Mondin V, Rinaldo A, Ferlito A. Management of nasal bone fractures. Am J Otolaryngol. 2005;26(3):181–5. doi: 10.1016/j.amjoto.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Al-Moraissi EA, Ellis E, 3rd. Local versus general anesthesia for the management of nasal bone fractures: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2015;73(4):606–15. doi: 10.1016/j.joms.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Javaherforooshzadeh F, Amirpour I, Janatmakan F, Soltanzadeh M. Comparison of effects of melatonin and gabapentin on post operative anxiety and pain in lumbar spine surgery: A randomized clinical trial. Anesthesiology and pain medicine. 2018;8(3) doi: 10.5812/aapm.68763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesioonpour S, Behaeen K, Dehghani Firoozabadi M. Effects of gabapentin on acute pain after nasal septoplasty. Otorinolaringologia. 2014;30:65–9. [Google Scholar]
- 6.Imani F, Rahimzadeh P, Khademi H, Narimani Zamanabadi M, Sadegi. et al Comparison of Transforaminal Triamcinolone and Dexmedetomidine in Radicular Low-Back Pain. Anesth Pain Med. 2019;9(5):e96117. Anesth Pain Med. 2019 doi: 10.5812/aapm.96117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javaherforooshzadeh F, Monajemzadeh SA, Soltanzadeh M, Janatmakan F, Salari A, Saeed H. A comparative study of the amount of bleeding and hemodynamic changes between dexmedetomidine infusion and remifentanil infusion for controlled hypotensive anesthesia in lumbar discopathy surgery: A double-blind, randomized, clinical trial. Anesthesiology and pain medicine. 2018;8(2) doi: 10.5812/aapm.66959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joana Afonso FR. Dexmedetomidine: Current Role in Anesthesia and Intensive Care. Rev Bras Anestesiol. 2012;62(1):118–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 9.Akhondzadeh R, Rashidi M, Gousheh M, Olapour A, Baniahmad A. The Effect of Adding Dexmedetomidine as an Adjuvant to Lidocaine in Forearm Fracture Surgeries by Supraclavicular Block Procedure Under Ultrasound-Guided. Anesth Pain Med. 2018;8(4):e74355. doi: 10.5812/aapm.74355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, Arslan G. Comparison of local anaesthesia with dexmedetomidine sedation and general anaesthesia during septoplasty. Eur J Anaesthesiol. 2010;27(11):960–4. doi: 10.1097/EJA.0b013e32833a45c4. [DOI] [PubMed] [Google Scholar]
- 11.Gousheh M, Akhondzadeh R, Rashidi M, Olapour A, Moftakhar F. Comparison of Dexmedetomidine and Morphine as Adjuvants to Bupivacaine for Epidural Anesthesia in Leg Fracture Surgery: A Randomized Clinical Trial. Anesth Pain Med. 2019;9(4):e91480. doi: 10.5812/aapm.91480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imani F, Rahimzadeh P, Faiz H, Nowruzina S, Shakeri A, Ghahremani M. Comparison of the Post-Caesarean Analgesic Effect of Adding Dexmedetomidine to Paracetamol and Ketorolac: A Randomized Clinical Trial. Anesth Pain Med. 2018;8(5):e85311. doi: 10.5812/aapm.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Therapeutics and Clinical Risk Management. 2016;(6):111–121. doi: 10.2147/tcrm.s5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahimzadeh P, Faiz SHR, Imani F, Derakhshan P, Amniati S. Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiol. 2018 ;18(1):62. doi: 10.1186/s12871-018-0531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imani F, Faiz HR, Sedaghat M, Hajiashrafi M. Effects of Adding Ketamine to Fentanyl Plus Acetaminophen on Postoperative Pain by Patient Controlled Analgesia in Abdominal Surgery. Anesth Pain Med. 2014;4(1):e12162. doi: 10.5812/aapm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imani F, Varrassi G. Ketamine as Adjuvant for Acute Pain Management. Anesth Pain Med. 2019;9(6):e100178. doi: 10.5812/aapm.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White PF, Coe V, Shafer A. Comparison of alfentanil with fentanyl for outpatient anesthesia. Anesthesiology and pain medicine. 1986;64:99–106. doi: 10.1097/00000542-198601000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–89. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg A, Kamal M, Mohammed S, Singariya G, Chouhan DS, Biyani G. Efficacy of dexmedetomidine for prevention of emergence agitation in patients posted for nasal surgery under desflurane anaesthesia: A prospective double-blinded randomised controlled trial. Indian J Anaesth. 2018;62(7):524–30. doi: 10.4103/ija.IJA_788_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56(6):542–6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jae-Wook J, Yong Han K, Ki Hwa L. Effect of dexmedetomidine on emergence agitation in male patients undergoing closed reduction of a nasal bone fracture. Rawal Medicine Journal. 2015;40(2) [Google Scholar]
- 22.Kim JC, Kim J, Kwak H, Ahn SW. Premedication with dexmedetomidine to reduce emergence agitation: a randomized controlled trial. BMC Anesthesiol. 2019;19(1):144. doi: 10.1186/s12871-019-0816-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111(2):222–8. doi: 10.1093/bja/aet056. [DOI] [PubMed] [Google Scholar]
- 24.Lee K, Yoo BH, Yon JH, Kim KM, Kim MC, Lee WY, et al. General anesthesia versus monitored anesthetic care with dexmedetomidine for closed reduction of nasal bone fracture. Korean J Anesthesiol. 2013;65(3):209–14. doi: 10.4097/kjae.2013.65.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]