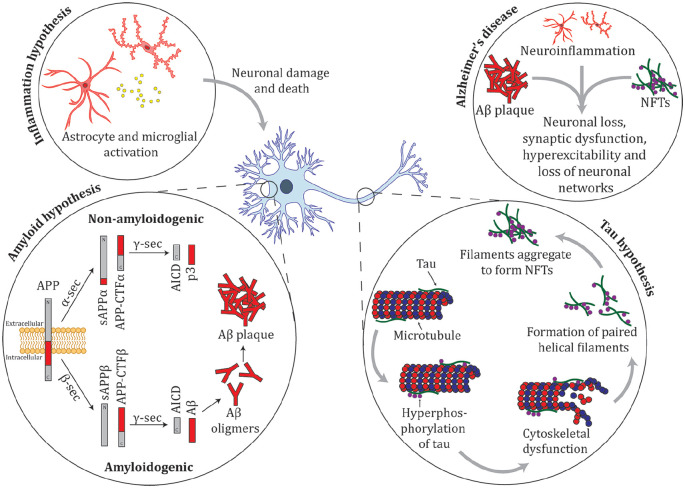
Figure 4.
Summary of the amyloid, tau, and inflammation hypotheses for Alzheimer’s disease. Amyloid precursor protein (APP) is a membrane protein that is proteolyticaly cleaved by multiple enzymes. The non-amyloidgenic pathway proceeds when APP is cleaved by the activity of α-secretase (α-sec) to soluble APP α (sAPPα) and APP carboxy terminal fragment α (APP-CTFα), these products are cleaved by γ-secretase (γ-sec) to produce truncated Aβ (p3) and the cytoplasmic polypeptide named AICD. The amyloidgenic pathway occurs when APP undergoes cleavage by β-secretase (β-sec) to form sAPPβ and APP-CTFβ. These proteins are cleaved by γ-sec to form AICD and amyloid-β (Aβ). In YOAD, mutations in APP, PSEN1, and PSEN2 there is an increase in the 42-residue Aβ42, relative to Aβ40, which leads to an increase in Aβ oligomers and amyloid plaque formation (Thinakaran and Koo 2008). Tau binds to microtubules and is important in cytoskeletal function. In AD, tau is hyperphosphorylated resulting in cytoskeletal dysfunction. Hyperphosphorylated tau detaches from microtubules and forms paired helical filaments that aggregate and form neurofibrillary tangles (NFTs). Inflammation is hypothesized to contribute to cognitive loss in AD. Astrocytes and microglia are activated in the AD brain, releasing pro-inflammatory cytokines (e.g., tumor necrosis factor-α [TNFα], interleukin [IL]-1β, IL-6, interferon-γ [IFNγ]) and chemokines (e.g., MIP-1α and MIP-1β) resulting in neuronal death, either by directly damaging neurons or by failing in their normal function to clear aggregates from the brain (Azizi and others 2015). There are multiple underlying molecular pathways leading to AD; intersecting pathways between amyloid, tau, inflammation, and other processes contribute to a complex mechanism that drives neurodegeneration.