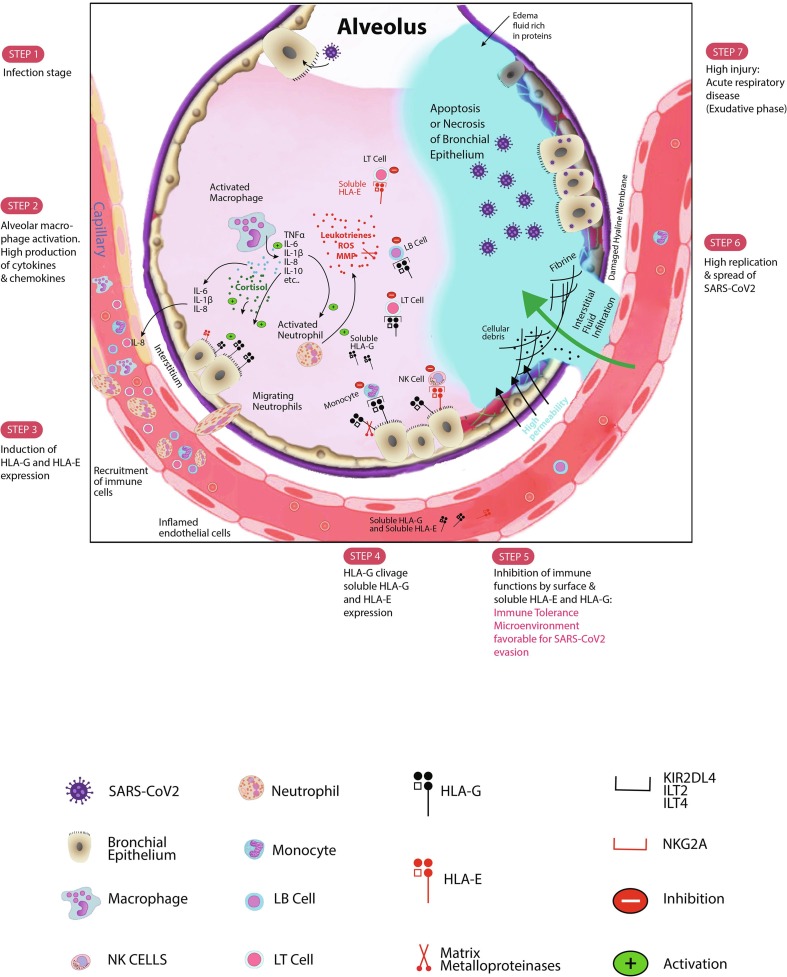
Fig. 1.
Schematic SARS-CoV2 infection evolution steps. After infecting bronchial epithelial cells, SARS-CoV2 decreases the IFN-γ production. Activated macrophages and monocytes particularly produce high levels of cytokines, chemokines, and also growth factors. Some cytokines and chemokines including IL-6, IL-1β, and IL-8 recruit immune cells to the site of SARS-CoV2 infection. The released IL-10, an anti-inflammatory cytokine, is produced to counteract the exacerbated inflammation. IL-10 might then stimulate the expression of HLA-G molecule at the surface of infected cells as well as microenvironment immune cells. Membrane-bound HLA-G strongly binds its inhibitory receptors on immune cells (NK, CD8+ T cells, LB cells, monocytes/dendritic cells) to disrupt their functions inducing immune system inhibition. This peripheral tolerance might be in favour of SARS-CoV2 subversion allowing high replication. Moreover, the probable presence of cortisol might stimulate both HLA-G production and lymphopenia. The activation of Neutrophil cells will increase production of leukotrienes, reactive oxygen species (ROS) and matrix metalloproteinases (MMP) causing pneumocytes and endothelial cells injury. The high injury will promote the constitution of the edema fluid causing the acute respiratory distress syndrome (ARDS). The production of MMP triggers the clivage of HLA-G and its release in bronchial cells microenvironment and in plasma that empower the inhibition of immune cells functions. Furthermore, HLA-E molecule either in its membrane-bound form or in its soluble form might downregulate synergically the immune cells functions. Soluble HLA-G/HLA-E together with membrane-bound HLA-G/HLA-E production may be detrimental for the SARS-CoV2 infected host. Along with current results and our proposed mechanism, we think that targeting these checkpoint molecules and/or their specific receptors will impair considerably the SARS-CoV2 progression.