Table 1.
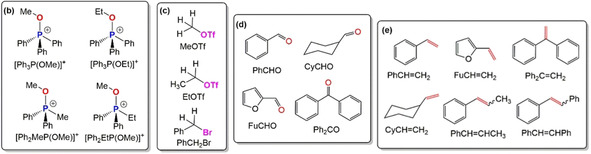
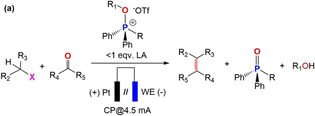
Screening of reaction conditions. (a) One‐pot reaction scheme for electrochemical WOR using [Ph2RP(OR1)]+ (R=Ph, Me, Et and R1=Me, Et) in the presence of substoichiometric amounts of Lewis acid (LA), (b) different [Ph2RP(OR1)]+, (c) alkyl electrophiles RX, (d) carbonyl compounds, and (e) olefins produced by electrochemical WOR.
|
| |||||||
|---|---|---|---|---|---|---|---|
|
Entry[a] |
WE[b] |
LA[c] |
[Ph2RP(OR1)]+[d] |
Alkyl electrophile[e] |
Carbonyl compound[f] |
Olefin |
Yield |
|
1 |
Ag |
– |
[Ph3P(OMe)]+ |
MeOTf |
PhCHO |
PhCH=CH2 |
<10 % |
|
2 |
Agg |
Sc3+ |
[Ph3P(OMe)]+ |
MeOTf |
PhCHO |
PhCH=CH2 |
63 % |
|
3 |
GCh |
Sc3+ |
[Ph3P(OMe)]+ |
MeOTf |
PhCHO |
PhCH=CH2 |
41 % |
|
4 |
Ag |
Yb3+i |
[Ph3P(OMe)]+ |
MeOTf |
PhCHO |
PhCH=CH2 |
33 % |
|
5 |
Ag |
Sc3+ |
[Ph2MeP(OMe)]+ |
MeOTf |
PhCHO |
PhCH=CH2 |
31 % |
|
6 |
Ag |
Sc3+ |
[Ph2EtP(OMe)]+j |
MeOTf |
PhCHO |
PhCH=CH2 |
– |
|
7 |
Ag |
Sc3+ |
[Ph3P(OEt)]+ |
EtOTf |
PhCHO |
PhCH=CHCH3 |
67 % |
|
8 |
Ag |
Sc3+ |
[Ph3P(OMe)]+ |
PhCH2Br |
PhCHO |
PhCH=CHPh |
43 % |
|
9 |
Ag |
Sc3+ |
[Ph3P(OMe)]+ |
MeOTf |
CyCHO |
CyCH=CH2 |
57 % |
|
10 |
Ag |
Sc3+ |
[Ph3P(OMe)]+ |
MeOTf |
FuCHO |
FuCH=CH2 |
46 % |
|
11 |
Ag |
Sc3+ |
[Ph3P(OMe)]+ |
MeOTf |
Ph2CO |
Ph2C=CH2 |
22 % |
[a] Reaction conditions: all the experiments were conducted under N2 atmosphere (glove box), 0.175 m TBAPF6 as supporting electrolyte, dried CH3CN prior to use as solvent (total volume 3 mL), electrolysis in separated (by glass frit) two electrode cell setup at a constant current (chronopotentiometry; CP at 4.5 mA) using a Pt foil as counter electrode (0.5×0.5 cm working area). [b] working electrode (WE) (0.5×0.5 cm working area). [c] highest yield of TPP and WOR was achieved with 0.02 m (0.6 equiv) LA. [d] electrolysis (2 h) was conducted with 0.032 m [Ph2RP(OR1)]+ in the presence of 0.6 equiv LA and formation of TPP was confirmed analyzing the solution by 31P(1H) NMR. [e] 0.048 m alkyl halide was added to the working compartment after 2 h of electrolysis and stirred for additional 30 minutes to prepare the phosphonium salt in situ. [f] 0.048 m aldehyde and/or ketone added further to the working compartment. [g] Ag foil 0.1 mm thick connected to with copper wire and copper tape (under an identical condition Cu foil, Mg foil and Ni foam did not produce alkene), [h] glassy carbon rod (GC; 6 mm diameter). [i] 0.02 m Yb(OTf)3; other LA (Fe3+, Ni2+, Zn2+ and B(OPh)3) remain ineffective for one‐pot WOR. [j] [R3POMe](OTf) (R=nBu and n‐oct) did not produce any olefin due to poor TPP regeneration (Figures S40 and S41).