Figure 2.
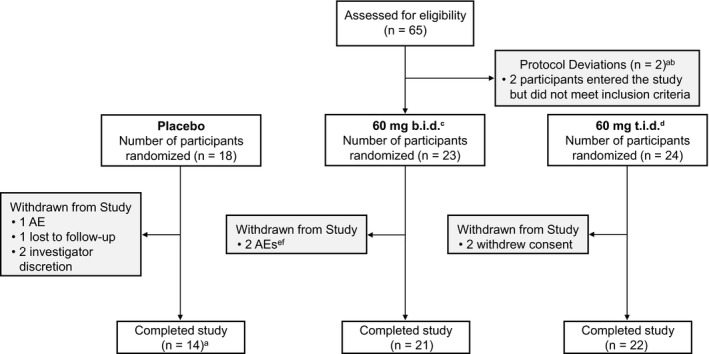
Patient disposition. aOne participant was found to have previously been exposed to 2 anti‐TNF biologic agents in the context of a previous clinical trial. The participant had already been dosed GSK2982772 and was therefore not withdrawn from the trial. bOne participant did not comply with contraception requirements. This was discovered on day 85, and the participant was therefore not withdrawn from the trial. cOne participant, randomized to the placebo group, erroneously received GSK2982772 on the day 29 visit. The participant was included in the 60‐mg b.i.d. treatment group. dOne participant was given captopril, a prohibited medication, for an SAE of hypertension on day 15 following a scheduled arthroscopic operation on the right knee. The participant was not withdrawn from the study as there were no safety concerns. eOne participant died following an accidental overdose of methylenedioxymethamphetamine 39 days after the start of the trial. fOne participant developed a herpes zoster infection on day 9 and was withdrawn from the study. AE, adverse event; b.i.d., twice daily; t.i.d., three times daily; SAE, serious adverse event.
