Figure 1.
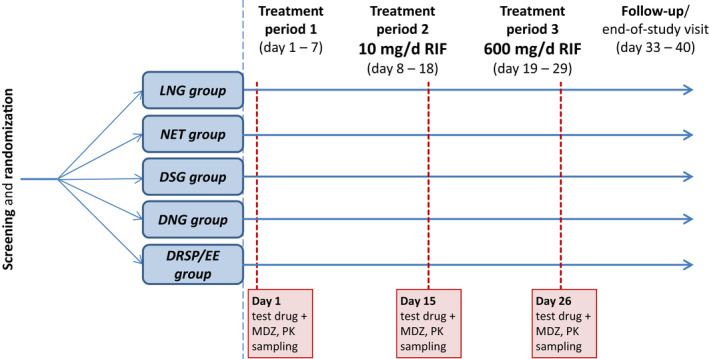
Study design. Treatment period 1 (control period): single administration of the randomly assigned test drug plus MDZ on day 1. Treatment period 2 (weak‐CYP3A‐induction period): administration of the randomly assigned test drug plus MDZ on day 15 was preceded and followed by once daily administration of rifampicin 10 mg for 7 and 4 days, respectively. Treatment period 3 (strong‐CYP3A‐induction period): administration of the randomly assigned test drug plus MDZ on day 26 was preceded and followed by once daily administration rifampicin 600 mg for 7 and 4 days, respectively. On days 15 and 26, rifampicin was administered 12 hours after the contraceptive and MDZ. There was no washout between low‐dose and high‐dose administration of rifampicin. The following test drugs (hormones) were co‐administered with MDZ 1 mg: LNG 0.03 mg; NET 0.35 mg; DSG 0.075 mg; DNG 2 mg; and DRSP 3 mg/EE 0.03 mg. Each treatment group included 12–14 subjects. DNG, dienogest; DRSP, drospirenone; DSG, desogestrel; EE, ethinylestradiol; LNG, levonorgestrel; MDZ, midazolam; NET, norethindrone; PK, pharmacokinetic; RIF, Rifampicin.
