Figure 2.
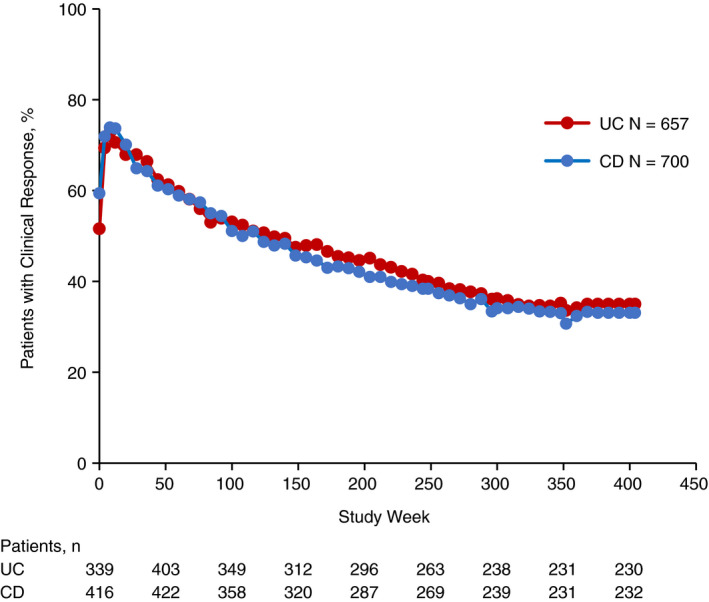
Clinical response over time in patients with UC or CD. Clinical response was assessed at baseline, weeks 4, 8, 12 and every 8 weeks thereafter. For patients with UC, clinical response was defined as a decrease in the partial Mayo score of ≥2 points and ≥25% from baseline, with an accompanying decrease in rectal bleeding subscore of ≥1 point from baseline or absolute rectal bleeding subscore of ≤1 point. For patients with CD, clinical response was defined as a ≥3‐point decrease from baseline in the HBI score. Baseline was defined as the last assessment prior to the first dose of study drug administration in GEMINI 1 for patients with UC and GEMINI 2 for patients with CD. For patients who discontinued the study, the final assessed value of clinical response was carried forward to the end of the study. CD, Crohn's disease; HBI, Harvey‐Bradshaw Index; UC, ulcerative colitis
