Figure 1.
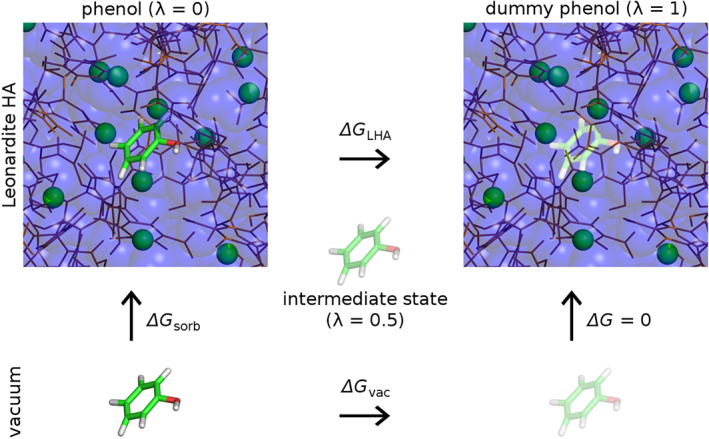
Sorption free energy of phenol in the Leonardite humic acid (LHA) model expressed as the difference in the solvation free energies in the LHA and vacuum (ΔG sorb = ΔG vac ‐ ΔG LHA) according to the thermodynamic cycle, as the sum of the free energy changes along the cycle is zero. Representative model of the LHA (top) with embedded phenol in its completely interacting form (λ = 0, left) and non‐interacting, dummy form (λ = 0, right). Horizontal arrows represent perturbation processes, for which the free energy changes are calculated. Water is shown in blue transparent spheres, LHA molecules in brown and line representation, and Ca ions as green spheres. Phenol is shown in stick representation, with the transparency depicting the level of perturbation
