FIGURE 1.
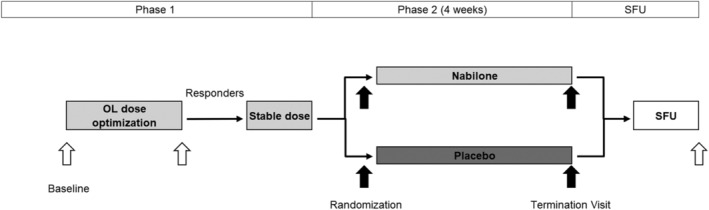
Schedule of trial activities. All patients received nabilone during phase I of the trial. The mean durations of phase I (including the open‐label titration phase and open‐label phase with stable nabilone dosage) and phase II (ie, double‐blind withdrawal phase) were 39.90 days ± 12.10 (median 37.00 days) and 28.37 days ± 3.23 (median 28.00 days), respectively. OL, open‐label; SFU, safety follow‐up.
