Abstract
Severe hypoglycaemia (SH) remains a challenge to people with type 1 diabetes (T1DM), and new‐generation basal insulins may improve patient outcomes. This post hoc meta‐analysis explored the risk of SH with insulin glargine 300 U/mL (Gla‐300) versus glargine 100 U/mL (Gla‐100) in a pooled population with T1DM from three randomized, multicentre, 6‐month similarly designed phase 3 trials: EDITION 4, EDITION JP 1 and EDITION JUNIOR. Endpoints included incidence and time to first occurrence of SH. Among 629 and 626 participants randomized to Gla‐300 and Gla‐100, respectively, glycated haemoglobin reductions were similar. Fewer participants experienced ≥1 SH event with Gla‐300 (6.2%) than with Gla‐100 (9.3%). From baseline to month 6, the risk of a first SH event was lower with Gla‐300: hazard ratio 0.65 [95% confidence interval (CI) 0.44–0.98; stratified log‐rank test P = 0.038]. SH event rates were numerically lower with Gla‐300 versus Gla‐100 from baseline to month 6 [relative risk (RR) 0.80 (95% CI 0.49–1.29); P = 0.356] and baseline to week 8 [RR 0.73 (95% CI 0.37–1.44); P = 0.369]. Thus, Gla‐300 demonstrated similar glycaemic control with lower risk of SH versus Gla‐100, particularly during the titration period.
Keywords: basal insulin, glycaemic control, hypoglycaemia, insulin analogues, meta‐analysis, type 1 diabetes
1. INTRODUCTION
Intensive insulin therapy to maintain glycaemic targets and reduce vascular complications exposes patients to increased risk of hypoglycaemia 1 and its negative consequences. 2 , 3 New‐generation basal insulins may reduce the risk of hypoglycaemia and improve glycaemic control.
Insulin glargine 300 U/mL (Gla‐300) is a second‐generation basal insulin analogue that provides more evenly distributed pharmacokinetic and pharmacodynamic profiles beyond 24 hours, compared with the first‐generation insulin glargine 100 U/mL (Gla‐100). 4 , 5 , 6 In clinical trials, Gla‐300 and Gla‐100 provided comparable glucose reductions, but Gla‐300 is associated with lower within‐day variability, improved suppression of plasma glucagon and lipid metabolism, and reduced rates of hypoglycaemia, despite greater insulin doses. 5 , 6 , 7
The efficacy and safety of Gla‐300 versus Gla‐100 in participants with type 1 diabetes (T1DM) were investigated in three phase 3, randomized, open‐label trials involving international (EDITION 4), 8 Japanese (EDITION JP 1) 9 and paediatric (EDITION JUNIOR) 10 populations. All studies had similar trial designs and demonstrated non‐inferiority in glycated haemoglobin (HbA1c) reduction with Gla‐300 versus Gla‐100, while numerically fewer patients experienced severe hypoglycaemia (SH) with Gla‐300 than with Gla‐100, during the main 6‐month treatment period. 8 , 9 , 10
This post hoc meta‐analysis aimed to further explore the risk of SH with initiation of Gla‐300 versus Gla‐100 in adults, children and adolescents with T1DM, who participated in the three pivotal EDITION studies.
2. METHODS
This meta‐analysis evaluated pooled data from participants in EDITION 4 (NCT01683266), EDITION JP 1 (NCT01689129) and EDITION JUNIOR (NCT02735044), which were multicentre, randomized, open‐label, parallel‐group phase 3 studies involving participants with T1DM, as described previously and in the Supplementary Methods in Appendix S1. 8 , 9 , 10 , 11
All studies had the same primary endpoint: change in HbA1c from baseline to week 26. In EDITION 4 and EDITION JP 1, participants were aged ≥18 years with HbA1c ≥53 to ≥7.0%–≤10% (≤86 mmol/mol) and had received basal plus prandial insulin for ≥1 year. In EDITION JUNIOR, participants were aged 6 to 17 years with T1DM for ≥1 year and had received basal plus prandial insulin for ≥6 months, with HbA1c ≥58 to ≥7.5%–≤11% (≤97 mmol/mol).
Participants followed a multiple daily injection regimen with basal plus prandial insulin over 26 weeks (Figure S1 in Appendix S1), titrated to a pre‐breakfast self‐monitored plasma glucose (SMPG) targets of 4.4 to 7.2 mmol/L (80–130 mg/dL) in EDITION 4 and EDITION JP 1 and 5.0 to 7.2 mmol/L (90–130 mg/dL) in EDITION JUNIOR (Table S1 in Appendix S1). 8 , 9 , 10 Participants continued their previous prandial insulin regimen, titrated at the investigator's discretion.
Efficacy endpoints for this post hoc meta‐analysis were change from baseline to week 26 in mean HbA1c, mean laboratory‐measured fasting plasma glucose and mean pre‐breakfast SMPG levels. Safety endpoints included incidence and event rates of SH from baseline to month 6 (main treatment period), baseline to week 8 (titration period), and week 9 to month 6 (maintenance period). Changes in mean insulin dose and mean body weight from baseline to week 26 were also analysed, along with adverse events (AEs), including incidence of diabetic ketoacidosis and serious adverse events (SAEs).
In adults, SH was defined as a hypoglycaemic event that required the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. In children/adolescents, SH was defined as an altered mental status and inability to assist in their care, being semi‐conscious or unconscious, or in a coma with or without convulsions that may require parenteral therapy (glucagon and/or glucose). SH events were assessed in all three clinical trials as part of the safety endpoints, which were pre‐specified and reported continuously throughout the study, along with AEs.
In the meta‐analysis, non‐inferiority of Gla‐300 versus Gla‐100 for HbA1c reduction was defined as the upper bound of the 95% confidence interval (CI) 0.3% (<3.3. mmol/mol) for the mean difference in HbA1c. Efficacy and safety data observed during the main 6‐month treatment period were included in the analyses.
Efficacy analyses were performed on the efficacy population as defined in each study (Supplementary Methods). Safety analyses were performed on the safety population, which included all randomized participants who received ≥1 dose of study insulin.
Continuous endpoints (eg, body weight, insulin dose, pre‐breakfast SMPG, fasting plasma glucose and HbA1c) were analysed using a mixed‐effect model for repeated measurements adjusted on treatment, visit, treatment‐by‐visit interaction, baseline value and baseline‐by‐visit interaction, and by adding fixed effects of study and study‐by‐visit interaction for the T1DM study pool.
Incidences of participants with ≥1 SH event were compared between treatment groups using an odds ratio based on a logistic model with treatment as fixed effect, and by adding the study as fixed effect for the T1DM study pool. The time to first event was assessed via a hazard ratio estimated using a Cox proportional hazard model with treatment group and study as fixed effects; log‐rank test was stratified by study, and cumulative incidence curves were calculated using Kaplan–Meier estimates. Event rates were compared using relative risk (RR) based on a negative binomial model with treatment as a fixed effect, logarithm of the treatment‐emergent period as offset, and by adding the study as a fixed effect for the T1DM study pool; the cumulative curves of number of events were calculated using Nelson–Aalen estimates. AEs were analysed descriptively using number and percentage of participants.
3. RESULTS
Overall, 629 participants were randomized to Gla‐300 treatment (EDITION 4, n = 274; EDITION JP 1, n = 122; EDITION JUNIOR, n = 233), and 626 participants to Gla‐100 treatment [EDITION 4, n = 275; EDITION JP 1, n = 121; EDITION JUNIOR, n = 230 (Figure S1)]. The efficacy population included 628 and 624 participants in the Gla‐300 and Gla‐100 group; the safety population included 629 and 624 participants in the Gla‐300 and Gla‐100 groups, respectively. The number of participants who discontinued treatment during the 6‐month treatment period was similar between groups (Table S2).
Baseline characteristics in the pooled randomized population were generally balanced between treatment groups (Table S3). The majority were adults and white, ~25% were Asian; approximately one‐third of participants were children/adolescents. Most used Gla‐100 as their basal insulin before study entry. The mean standard deviation HbA1c level at baseline was 8.3 (0.8)% in both groups.
Reductions in HbA1c from baseline to week 26 were similar in both groups (Figure S2A). The least squares (LS) mean difference in HbA1c between Gla‐300 and Gla‐100 was 0.05 (95% CI −0.044 to 0.150)%, demonstrating non‐inferiority of Gla‐300 versus Gla‐100 (Figure S2B). While pre‐breakfast SMPG initially increased in the Gla‐300 group, overall change was similar between the treatment groups from baseline to week 26 (Figure S3). The LS mean (SE) change in fasting plasma glucose was −0.84 (0.197) mmol/L in the Gla‐300 group and − 1.09 (0.197) mmol/L in the Gla‐100 group. Body weight increased in both groups (Figure S4).
The incidence of SH with Gla‐300 versus Gla‐100 was not significantly different across the individual EDITION studies, although SH was numerically lower with Gla‐300 (Figure 1). In the pooled analysis, significantly fewer participants experienced ≥1 SH event from baseline to month 6 with Gla‐300 [39 (6.2%)] than Gla‐100 [58 (9.3%); hazard ratio 0.65 (95% CI 0.44 to 0.98); stratified log‐rank test P = 0.038 (Figure 1A)]. The odds ratio of ≥1 SH event with Gla‐300 versus Gla‐100 in the study pool was significant between baseline and month 6 [0.65 (95% CI 0.42 to 0.98); P = 0.042], and between baseline and week 8 [0.50 (95% CI 0.27 to 0.95); P = 0.033], but not significant between week 9 and month 6 [0.86 (95% CI 0.51 to 1.45); P = 0.573 (Figure 1B)]. The incidence of SH was numerically lower with Gla‐300 versus Gla‐100 between 6:00 and 11:59 am (Figure S5).
FIGURE 1.
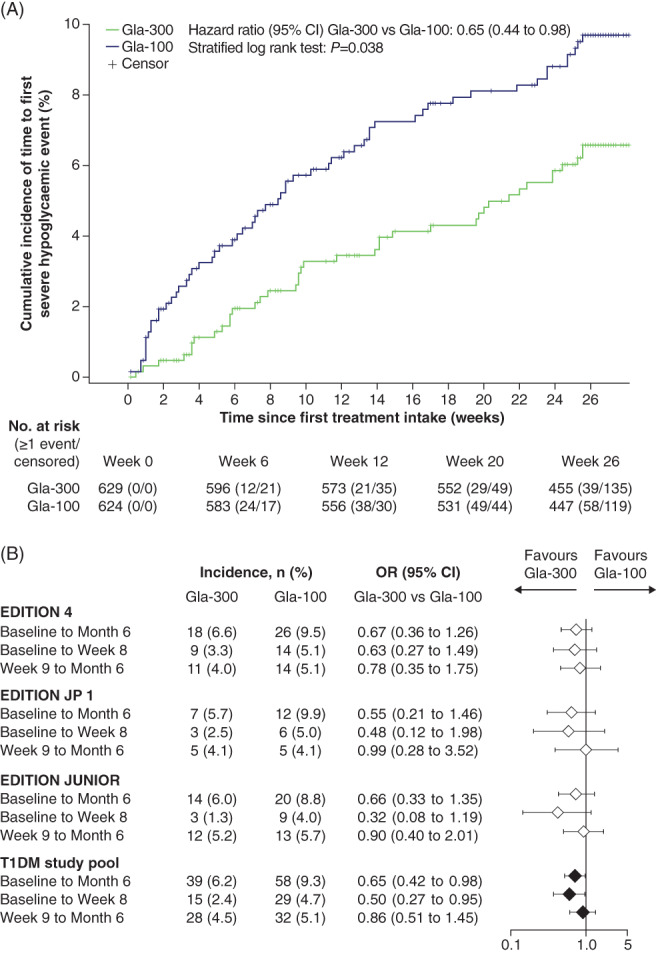
A, Kaplan–Meier plot showing cumulative incidence of the time to first severe hypoglycaemia (SH) event in participants with type 1 diabetes (T1DM) for the pooled studies. B, Incidence of SH for the individual studies and pooled studies during the main 6‐month treatment period (safety populations). Odds ratios (ORs) based on logistic models with treatment as fixed effect and by adding study as fixed effect for the T1DM pooled studies. CI, confidence interval; Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL
The number of SH events reported with Gla‐300 versus Gla‐100 from baseline to month 6 was not significantly different across the individual studies, or the pooled analysis; the number of SH events from baseline to month 6 was 70 (0.23 events/participant‐year) with Gla‐300 and 86 (0.29 events/participant‐year) with Gla‐100. The RR of Gla‐300 versus Gla‐100 was 0.80 (95% CI 0.49 to 1.29; P = 0.356) from baseline to month 6, 0.73 (95% CI 0.37 to 1.44; P = 0.369) from baseline to week 8, and 0.84 (95% CI 0.45 to 1.57; P = 0.590) from week 9 to month 6 (Figure 2).
FIGURE 2.
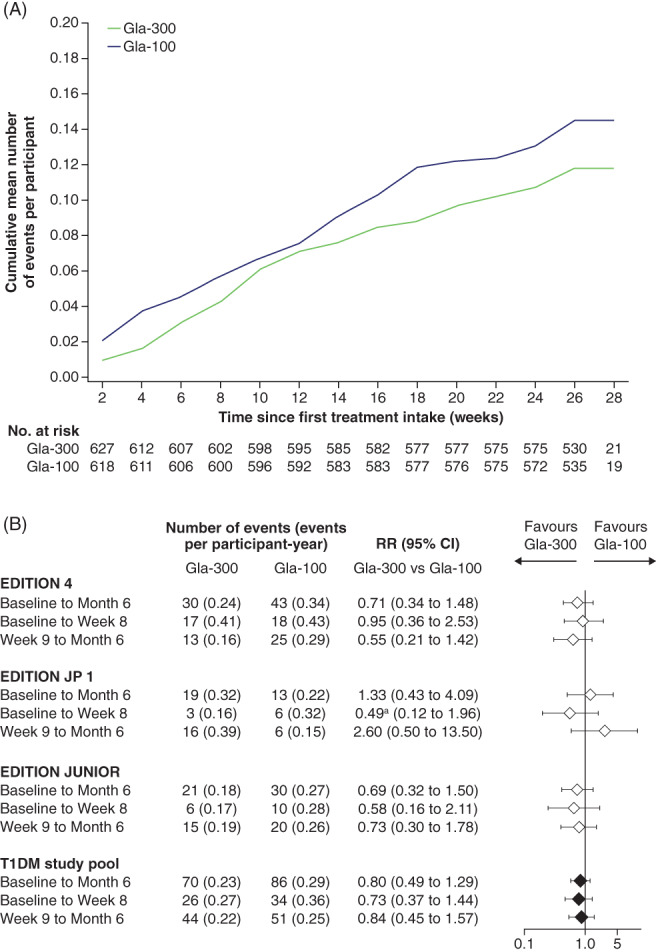
A, Cumulative mean number of events of severe hypoglycaemia (SH) per participant for the pooled studies. B, Event rates and relative risk (RR) of SH events for the individual studies and the pooled studies, during the main 6‐month treatment period (safety populations). aModel calculated with low number of events (3 vs. 6). RR based on negative binomial models with treatment as fixed effect, and logarithm of the treatment‐emergent period as offset, and by adding study as fixed effect for the type 1 diabetes (T1DM) pooled studies; cumulative mean number calculated using Nelson–Aalen estimates. CI, confidence interval; Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL
The basal insulin dose increased in both treatment groups from baseline [LS mean (SE) 0.36 (0.007) U/kg for Gla‐300 and 0.37 (0.007) U/kg for Gla‐100] to week 26 [LS mean (SE) 0.50 (0.006) U/kg for Gla‐300 and 0.43 (0.006) U/kg for Gla‐100 (Figure S3)]. The daily prandial insulin dose remained almost stable across the two groups, from baseline [LS mean (SE) 0.40 (0.009) U/kg for Gla‐300 and 0.39 (0.008) U/kg for Gla‐100] to week 26 [LS mean (SE) 0.41 (0.007) U/kg for Gla‐300 and 0.41 (0.007) U/kg for Gla‐100].
Overall, 395 (62.8%) and 388 participants (62.2%) experienced AEs in the Gla‐300 and Gla‐100 groups. Diabetic ketoacidosis occurred in two (0.3%) and five participants (0.8%), and SAEs were experienced by 37 (5.9%) and 46 participants (7.4%) in the Gla‐300 and Gla‐100 groups, respectively. Two deaths occurred in the Gla‐300 group: one from suicide, the other from a cardiac event; neither was considered related to study drug. The number of participants who discontinued treatment owing to AEs was 6 (1.0%) with Gla‐300 and 5 (0.8%) with Gla‐100.
4. DISCUSSION
In this patient‐level meta‐analysis of three EDITION studies, a significantly lower risk of SH with Gla‐300 versus Gla‐100 was observed from baseline to month 6, when data from the three clinical trials were pooled. This result was primarily driven by a lower SH risk during the first 8 weeks (Figure 1B), suggesting that Gla‐300 may be useful in limiting the risk of SH, especially during the phase of most active insulin titration. Indeed, the number of participants needed to be treated with Gla‐300 rather than Gla‐100 in order to prevent one participant experiencing SH was 45 in the first 8 weeks of treatment (titration period), and 33 in the overall 6‐month period, which is clinically meaningful compared with a number needed to treat of 148 from week 9 to month 6.
While the results of this analysis may be attributable to the increased power gained from analysing the pooled population with T1DM, it nonetheless underpins a clinically meaningful advantage with Gla‐300 versus Gla‐100 that was identifiable as a trend in the individual studies. Notably, the decreased risk of SH with Gla‐300 was achieved alongside similar HbA1c reductions of ~0.4%, and similar overall safety profiles, in both treatment groups.
The reduced SH risk with Gla‐300 versus Gla‐100 may be attributable to the more favourable pharmacokinetic/pharmacodynamic profile of Gla‐300 versus Gla‐100. 5 The smoother average 24‐hour glucose profile of Gla‐300 may also reduce glycaemic variability, irrespective of morning or evening injection, rendering it a more flexible basal insulin analogue than Gla‐100. 6 These more physiological characteristics of Gla‐300 may explain the reduction of SH in the early and late morning hours (6:00 to 11:59 am; Figure S5), in line with similar observations in studies of people with type 2 diabetes. 12 , 13 Notably, pre‐breakfast SMPG was slightly higher with Gla‐300 versus Gla‐100 initially following the switch to Gla‐300, despite greater doses of Gla‐300 (Figure S3). This suggests that a transient, slight increase of fasting glycaemic levels during titration with Gla‐300 may reduce the SH risk more effectively than Gla‐100, supported by the separation of the risk curves for Gla‐300 and Gla‐100 shortly after Gla‐300 treatment initiation (Figure 1A).
The findings of this meta‐analysis are consistent with those of real‐world analyses. In one retrospective study, the number of nocturnal hypoglycaemic events was significantly reduced within 2 weeks after patients with T1DM were switched from Gla‐100 to Gla‐300. 14 In the SPARTA study, the incidence of SH remained similar before and 6 months after initiation of Gla‐300, despite a significant HbA1c reduction of 0.4%. 15
This post hoc, exploratory analysis has some limitations. For example, it includes a heterogeneous population from the three EDITION studies; however, this population is also representative of a large multinational cohort of patients with T1DM. Other factors that may impact the occurrence of SH, such as changes in carbohydrate intake, physical activity and stress, were not accounted for in this meta‐analysis. Additionally, data collection pertaining to SH events was dependent on participant reporting; however, the prospective clinical trial setting with pre‐specified hypoglycaemia endpoints, an above‐average visit schedule and robust monitoring efforts may provide confidence in the reported results. Strengths of the analysis are the similarity between study designs and use of patient‐level data.
In summary, this meta‐analysis suggests Gla‐300 may hold a clinically important advantage over Gla‐100 with respect to fewer SH events, particularly during the titration period, while providing similarly improved glycaemic control in people with T1DM.
CONFLICTS OF INTEREST
T.D. has received research support or has consulted for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, DexCom, Insulet Corp., Eli Lilly, Medtronic, NovoNordisk and Roche, and is a shareholder of DreaMed Ltd. M.M. has received research support from Tanabe‐Mitsubishi, Novartis, Boehringer Ingelheim, Novo Nordisk, Takeda, Ono, Sysmex, Nissui and speaker's bureau from Sanofi, Novo Nordisk, Tanabe‐Mitsubishi, Takeda, Astellas, Eli Lilly, Merck Sharp & Dohme Corp. C.S. is an employee and shareholder of Sanofi. H.G. is an employee and shareholder of Sanofi. F.L. was an employee and shareholder of Sanofi at the time of manuscript preparation. E.N. is an employee and shareholder of Sanofi. G.B.B. has received honoraria or consulting fees from Menarini and Sanofi and has received research support/speaker's bureau from Sanofi.
AUTHOR CONTRIBUTIONS
Sanofi was the sponsor of the study and was responsible for the design and coordination of the trial, monitoring clinical sites, collecting and managing data, and performing all statistical analyses. All authors were involved in developing the initial concept for analysis and data acquisition. All authors contributed to interpreting the findings and writing, reviewing and editing the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This study was sponsored by Sanofi. The authors thank the study participants, trial staff and investigators for their participation. Editorial and writing assistance was provided by Jennina Taylor‐Wells, PhD, of Fishawack Communications Ltd., UK, and was funded by Sanofi.
Danne T, Matsuhisa M, Sussebach C, et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: A meta‐analysis of 6‐month phase 3 clinical trials. Diabetes Obes Metab. 2020;22:1880–1885. 10.1111/dom.14109
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14109.
Funding information The meta‐analysis and clinical trials considered in the analysis were sponsored by Sanofi, Paris, France. Editorial and writing assistance was provided by Jennina Taylor‐Wells, PhD, of Fishawack Communications Ltd., UK, and was funded by Sanofi.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient‐level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
REFERENCES
- 1. Nathan DM, DCCT Edic Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahola AJ, Saraheimo M, Freese R, et al. Fear of hypoglycaemia and self‐management in type 1 diabetes. J Clin Transl Endocrinol. 2016;4:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin‐treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units. mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units. mL‐1. Diabetes Care. 2015;38(4):637‐643. [DOI] [PubMed] [Google Scholar]
- 5. Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics, pharmacodynamics, and modulation of hepatic glucose production with insulin Glargine U300 and Glargine U100 at steady state with individualized clinical doses in type 1 diabetes. Diabetes Care. 2019;42(1):85‐92. [DOI] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40(4):554‐560. [DOI] [PubMed] [Google Scholar]
- 7. Lucidi P, Porcellati F, Cioli P, et al. Greater suppression of glucagon, lipolysis and ketogenesis with insulin glargine U300 as compared to glargine U100 in type 1 diabetes mellitus. Diabetes Technol Ther. 2020;22(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 8. Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care. 2015;38(12):2217‐2225. [DOI] [PubMed] [Google Scholar]
- 9. Matsuhisa M, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016;18(4):375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danne T, Tamborlane WV, Malievsky OA, et al. Efficacy and safety of insulin glargine 300 U/mL (Gla‐300) vs insulin glargine 100 U/mL (Gla‐100) in children and adolescents (6–17years) with type 1 diabetes: results of the EDITION JUNIOR randomized controlled trial. Diabetes Care. 2020;43(7):1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Home PD, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (EDITION 4). Diabetes Obes Metab. 2018;20(1):121‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riddle MC, Bolli GB, Avogaro A, et al. Assessment of hypoglycaemia during basal insulin therapy: temporal distribution and risk of events using a predefined or an expanded definition of nocturnal events. Diabetes Metab. 2018;44(4):333‐340. [DOI] [PubMed] [Google Scholar]
- 13. Bolli GB, Wysham C, Fisher M, et al. A post‐hoc pooled analysis to evaluate the risk of hypoglycaemia with insulin glargine 300 U/mL (Gla‐300) versus 100 U/mL (Gla‐100) over wider nocturnal windows in individuals with type 2 diabetes on a basal‐only insulin regimen. Diabetes Obes Metab. 2019;21(2):402‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oriot P, Jeremie W, Buysschaert M. Outcomes of glycemic control in type 1 diabetic patients switched from basal insulin glargine 100 U/ml to glargine 300 U/ml in real life. Expert Rev Endocrinol Metab. 2018;13(3):167‐171. [DOI] [PubMed] [Google Scholar]
- 15. Pang T, Bain SC, Black RNA, et al. A multicentre, UK, retrospective, observational study to assess the effectiveness of insulin glargine 300 units/ml in treating people with type 1 diabetes mellitus in routine clinical practice (SPARTA). Diabet Med. 2019;36(1):110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Qualified researchers may request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient‐level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.


