Figure 4.
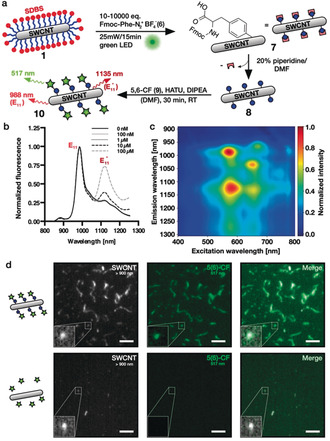
Fmoc‐phenylalanine quantum defects and multicolor SWCNTs. a) Strategy for defect introduction and subsequent Fmoc deprotection followed by CF conjugation. DIPEA=N,N‐diisopropylethylamine, HATU=1‐[bis(dimethylamino)methylene]‐1H‐1,2,3‐triazolo[4,5‐b]pyridinium 3‐oxide hexafluorophosphate. b) NIR fluorescence spectra of SWCNTs treated with different concentrations of Fmoc‐Phe‐Dz showing increased E11*/E11 ratios at higher diazonium salt concentrations. c) Excitation–emission map of Fmoc‐Phe‐SWCNT* showing the E11* fluorescence amongst other minor SWCNT species and E11 fluorescence. d) SWCNT*‐Phe‐CF immobilized on a glass slide showing colocalization of the NIR (>900 nm) and the CF channels (500–550 nm), whereas the control without sp3 defects does not show a CF signal, indicating successful conjugation. Scale bars=5 μm.
