Figure 5.
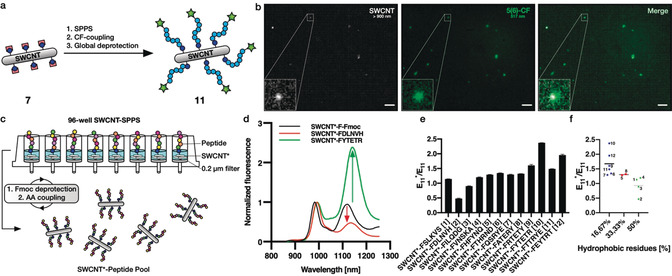
In situ peptide synthesis and modulation of E11* peak intensities. a) Strategy for the generation of covalent and fluorescent SWCNT*‐F‐R6‐CF conjugates based on Fmoc/OtBu‐solid‐phase peptide synthesis (SPPS) followed by N‐terminal CF coupling and Pbf deprotection of the arginine side chains. b) SWCNT*‐R6‐CF spin‐coated on a glass coverslip showing colocalization of the NIR and the CF channels, indicating successful peptide synthesis and N‐terminal CF coupling directly on the SWCNT sidewall (scale bars=10 μm). c) 96‐Well peptide synthesis for the generation of a SWCNT‐peptide pool following the same Fmoc/OtBu‐SPPS protocol as shown in (a), yet here in a 96‐well plate with filters (0.2 μm pore size). d) Normalized NIR‐PL spectra before and after synthesis of two selected peptide sequences showing the modulation of the defect‐induced fluorescence. The red‐shift from the protected to the unprotected sample could be attributed to the different surfactants (SWCNT*‐F‐Fmoc: SDBS, SWCNT*‐Peptide: DOC). e) The SWCNT fluorescence properties (in particular the E11*/E11 ratio) depends on peptide sequence on the sidewall (mean ± SD, n=3). f) E11*/E11 ratio increases with the number of hydrophobic residues (mean and individual values, n=6, 2, 4).
