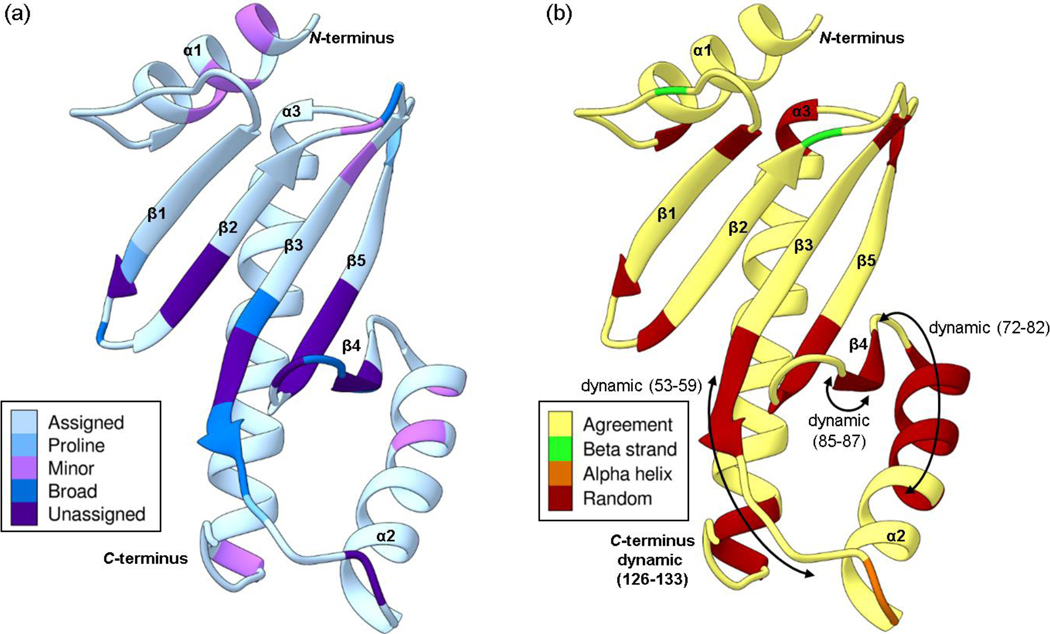
Fig. 3.
The Phyre2 structural homology model of Scc4 with the (a) assignment status and (b) secondary structure comparison. (a) The assigned residues are shaded in light blue, the prolines in medium blue, and the unassigned residues are in purple. Residues that show minor peaks in the 1H,15N-HSQC spectrum are shaded in pink, and residues with broad resonances are shaded in dark blue. (b) The secondary structure of the Phyre2 homology model is shown as a ribbon drawing. Residues shaded in yellow have NMR-determined secondary structure in agreement with the homology model. Residues with NMR-determined secondary structures that are different from the model are shaded in green for beta strands, orange for alpha helices, and red for random coil. The arrows indicate residues that are dynamic with RCI-S2 values < 0.7. The color schemes are based on palettes for color blindness and interpretability in grayscale (Krzywinski 2020).