Figure 1. Vaccination of RSV-experienced young mice elicits high RSV neutralizing antibody titers and protects against secondary infection.
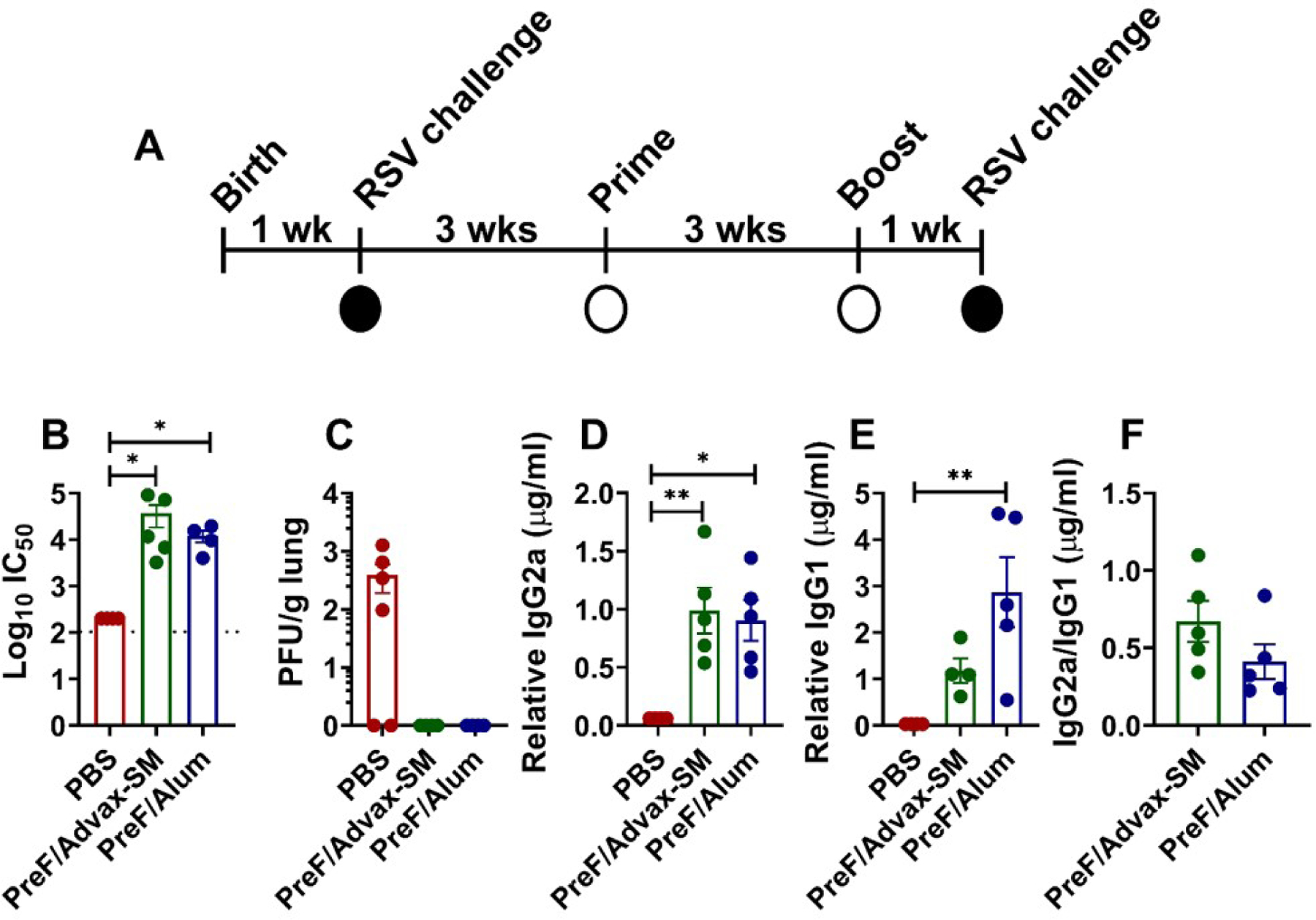
At 5 to 6 days of age, infant BALB/cJ mice were infected with 5x105 PFU/gram of RSV L19. Three weeks later, female mice were immunized with Prefusion RSV F protein (PreF)/Advax-SM, PreF/Alum, or mock-vaccinated with PBS, then boosted 3 weeks after initial immunization. One-week post-boost, mice were bled and subsequently challenged with 5x105 PFU/g of RSV L19. Mice were culled for sample collection at 4- or 8-days post-infection (dpi) (A). RSV neutralizing antibody levels (B), PreF-specific IgG2a (D), PreF-specific IgG1 (E), and IgG2a/IgG1 ratio (F) were obtained from pre-challenge serum. IgG2a/IgG1 ratio was determined by dividing PreF-specific relative IgG2a (µg/mL) by PreF-specific relative IgG1(µg/mL). Left lungs or upper right lungs were harvested to assess viral titers at 4dpi with quantification using H&E plaque assays (C). Viral titers were performed in triplicate and data within each group represent the mean titer for each animal. Data are represented as mean ± SEM. Statistical significance between vaccination groups was calculated using one-way ANOVA with Dunn’s multiple comparison post-test (B and C), one-way ANOVA with Tukey’s multiple comparison post-test (D and E), or unpaired t-test (F) between all groups. *p<0.05 and **p<0.01.
