Fig. 3. The TRD mediates DNA binding but prevents NCP core binding.
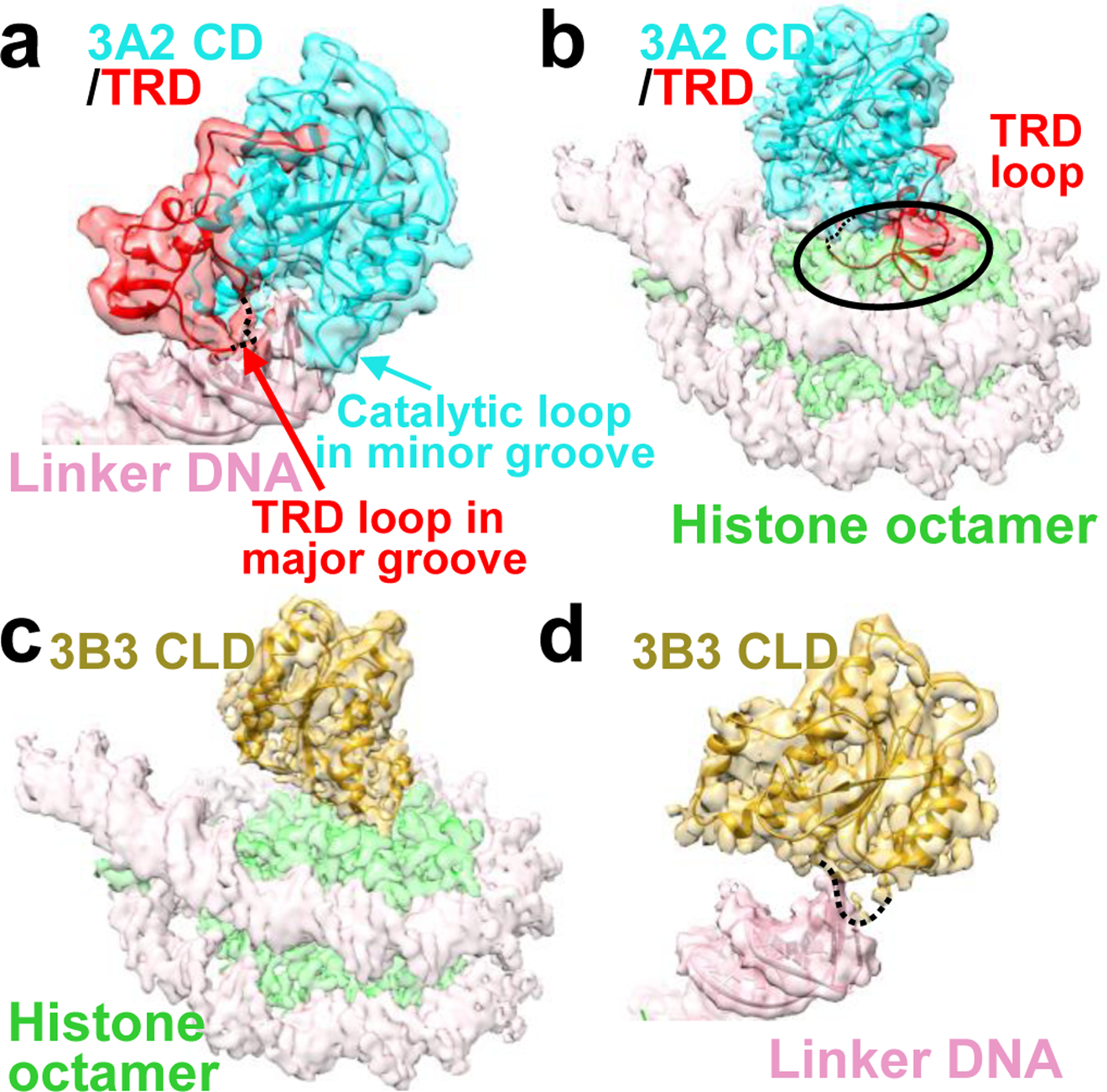
a, Binding of the DNMT3A2 catalytic domain (3A2 CD) to linker DNA. b, Structural alignment model in which the DNMT3B3 catalytic-like domain (3B3 CLD) bound to the histone octamer is replaced by the 3A2 CD. Note that the TRD clashes with the position of the histone octamer (indicated by the black oval), preventing NCP core binding. Dashed line: part of the TRD loop with weak density that has been deleted from the final model (see Ext. Data Fig. 4d). c, Binding of the DNMT3B3 catalytic-like domain (3B3 CLD) to the histone core. d, Model in which 3A2 CD bound to linker DNA is replaced by 3B3 CLD. Without the TRD, the 3B3 CLD only weakly interacts with linker DNA. Dashed line indicates the position of the catalytic loop. Transparent cryo-EM density generated in UCSF Chimera at 0.013 contour level overlaid with solid cartoon model.
