FIGURE 1.
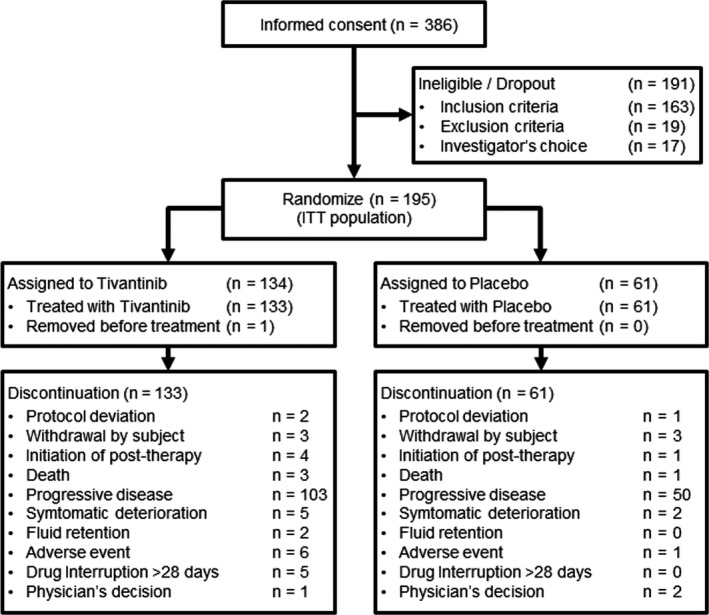
Summary of patient disposition. After providing consent, patients were screened via several tests, including a test of c‐Met expression in tumor samples. Eligible patients who had at least one tumor sample with MET‐high were randomly assigned to either the tivantinib group or the placebo group at a 2:1 ratio
