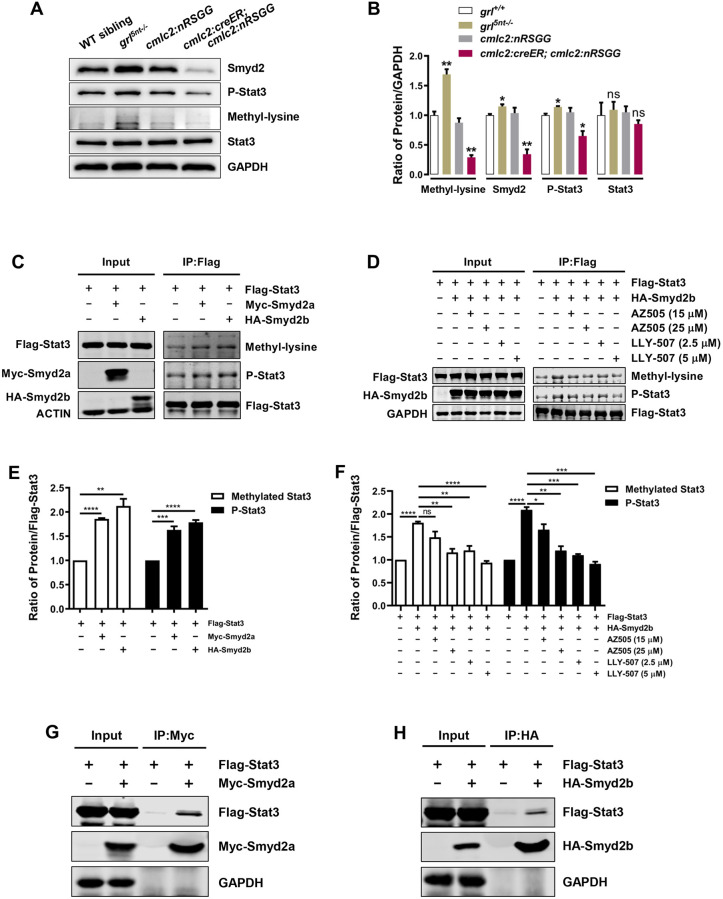
Fig. 6.
Smyd2 functions as a KMT and controls Stat3 methylation and phosphorylation. (A) western blot analysis exhibiting Smyd2, Stat3, P-Stat3 or corresponding lysine methylated proteins using total lysates extracted from WT sibling, grl5nt−/−, 4-HT-treated control Tg(cmlc2:nRSGG) hearts or 4-HT-treated Tg(cmlc2:creER;cmlc2:nRSGG) hearts at 7 dpa, respectively. GAPDH was used as a loading control. (B) Quantification of western blots using ImageJ software and normalized to GAPDH (n=3). (C) Methylation and phosphorylation assay showing increased levels of methylated Stat3 and P-Stat3 in Flag-Stat3 and Myc-Smyd2a/HA-Smyd2b co-transfected HEK 293T cells. The cell lysates were used to immunoprecipitate (IP) Stat3 with anti-Flag antibody and then blotted with anti-methyl-lysine or anti-P-Stat3 antibodies. (D) Treatment with AZ505 or LLY-507 diminishes Stat3 methylation and phosphorylation in cells co-transfected with Flag-Stat3 and HA-Smyd2b. The cell lysates were treated with AZ505 (15 µM for 6 h, 25 µM for 6 h) or LLY-507 (2.5 µM for 28 h, 5 µM for 28 h), used to IP Stat3 using anti-Flag antibody and then blotted with anti-methyl-lysine and anti-P-Stat3 antibodies. (E) Quantification of methylated Stat3 and phosphorylated Stat3 levels normalized to Flag-Stat3 (n=3). (F) Quantification of methylated Stat3 and phosphorylated Stat3 levels normalized to Flag-Stat3 in cells treated with AZ505 or LLY-507 (n=3). (G,H) Smyd2a or Smyd2b immunoprecipitation with anti-Myc antibody (G) or anti-HA antibody (H), respectively, detects Stat3 using anti-Flag antibody. HEK 293T cells were co-transfected with constructs of Flag-Stat3 and Myc-Smyd2a (G) or HA-Smyd2b (H). GAPDH was used as a loading control. Data represents mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Student's t-test (unpaired, two-tailed).