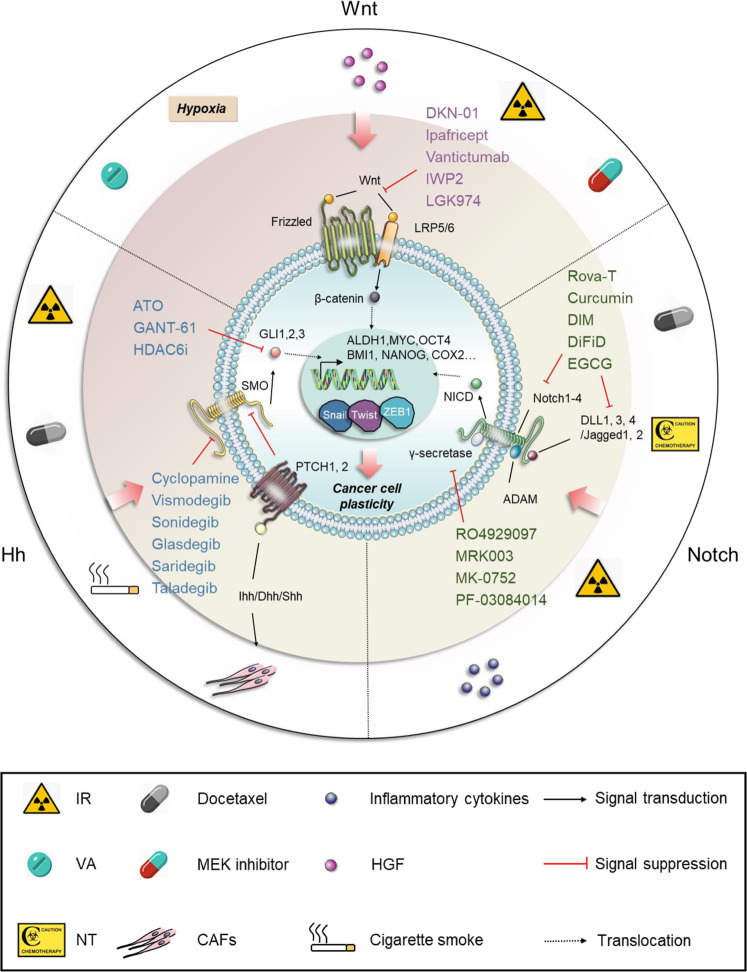
Fig. 7.
Overview of the molecular basis of re-activation of developmental programs contributing to cancer cell plasticity—major inhibitors of Hh, Wnt, and Notch signaling pathways for targeted therapy. The Hedgehog (Hh), wingless/integrated (Wnt), and Notch signaling pathways play a crucial part in acquisition and expansion of CSC phenotype after being stimulated by internal factors or extrinsic stimuli, for instance, HGF and docetaxel. Re-activation of these developmental programs promotes the corresponding transcription factors entering into the nucleus to regulate expression of downstream effectors that are closely related to CSCs regeneration and maintenance, as well as multiple biological functions. To prevent cancer cell plasticity, particularly phenotypic switching from non-CSC to CSC states induced by Hh, Wnt, and Notch signaling pathways, a range of inhibitors targeting these pathways have been approved for clinical use or are under development, as shown in blue, purple, and green. CAFs cancer-associated fibroblasts, Ihh/Dhh/Shh: Sonic hedgehog/Indian hedgehog/Desert hedgehog, PTCH1 2 patched1 and patched2, SMO smoothened, ATO arsenic trioxide, HDAC6i histone deacetylase 6 inhibitor, GLI glioma-associated oncogene homolog, LRP5/6 lipoprotein receptor-related protein 5/6, HGF hepatocyte growth factor, VA valproic acid, EMT epithelial–mesenchymal transition, NICD Notch intracellular region, ADAM a disintegrin and metalloprotease, DLL1, 3, 4 Delta-like ligand 1, 3, 4, Jagged 1, 2 Serrate-like ligand 1, 2, IR ionizing radiation, NT neoadjuvant therapy, Rova-T rovalpituzumab tesirine, DIM 3,3′-diindolylmethane, DiFiD 3,5-bis (2,4-difluorobenzylidene)-4-piperidone, EGCG epigallocatechin-3-gallate