Figure 5. miR-124 overexpression in vivo shows functional mitigation of RICD.
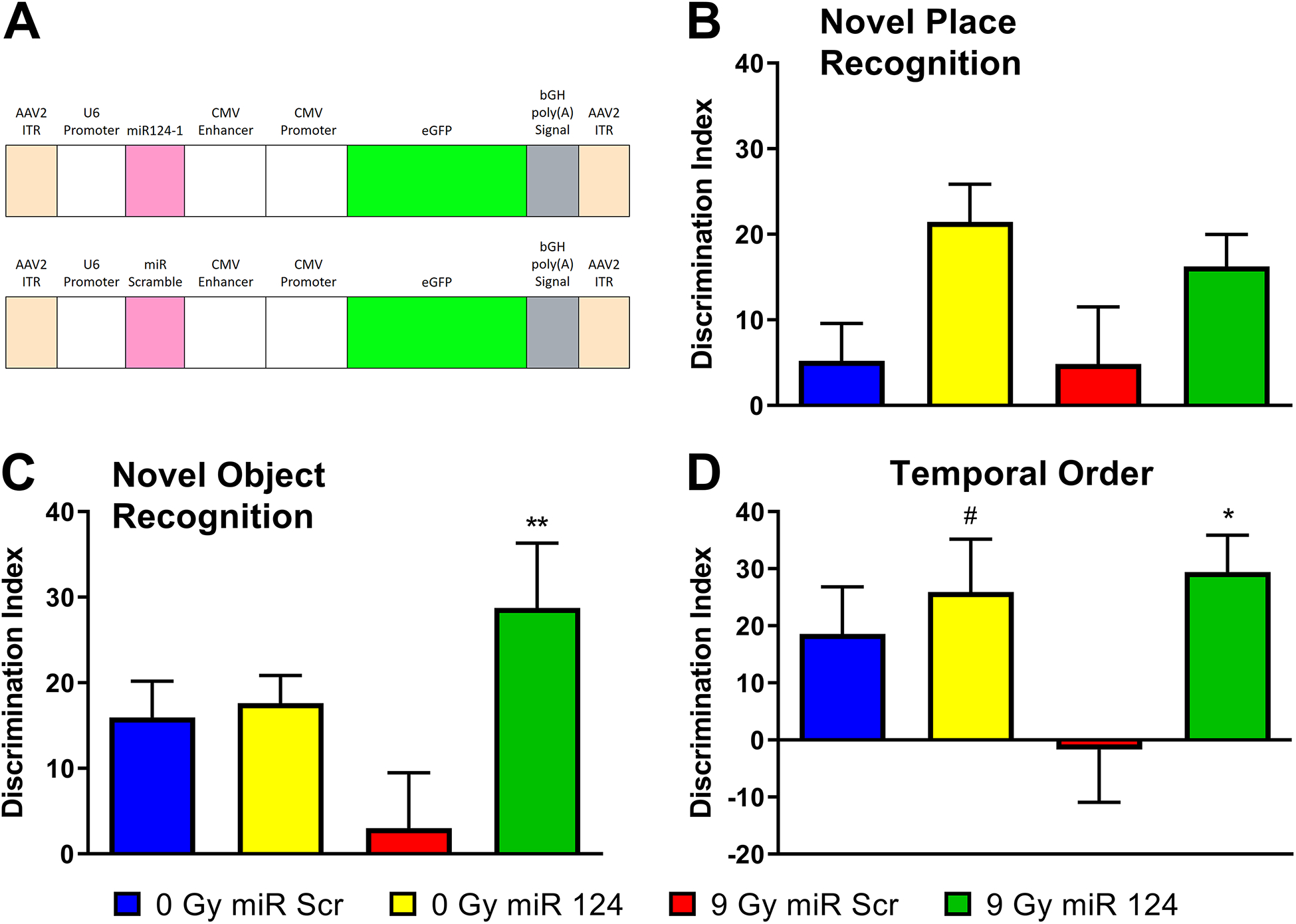
To determine whether miR-124 was sufficient to mitigate RICD in wild type mice, the miRNA sequence was cloned into a vector designed to overexpress miR-124 and the construct packaged into AAV9 particles. The vector map (A, designed by Signagen) shows that miR-124 expression is driven by the U6 promoter and eGFP expression is driven by the CMV enhancer and promoter. These transgenes are flanked by AAV2 inverted terminal repeats (ITR) for efficient propagation of the AAV genome. Mice received stereotaxic IC injections of AAV9 particles containing this vector two days post-irradiation. At five weeks post-irradiation, animals were administered spontaneous exploration tasks in the following order: NPR (B), NOR (C), and TO (D). Tendency to explore novelty (novel place or object) was calculated using the discrimination index [(novel location exploration time/total exploration time) – (familiar location exploration time/total exploration time)] × 100. All data are presented as mean ± SEM (N=10–11 mice per group). # P = 0.0724, * P < 0.05, ** P < 0.01 compared to the IRR group. P values are derived from one-way ANOVA and Dunnett’s test for multiple comparisons (all other groups compared to the 9 Gy + miR-Scr group).
