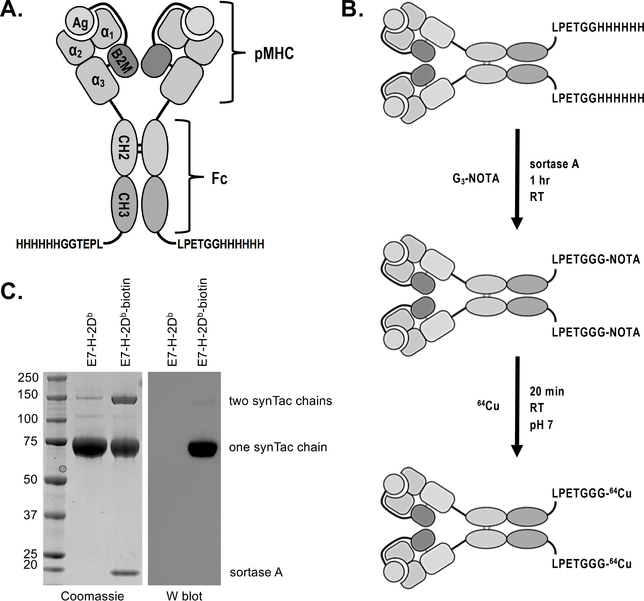
Figure 1.
SynTac design, validation, and labeling strategy. A) Two pMHC (H-2Db) molecules are covalently attached to an IgG2a Fc region. The antigen is expressed as a fusion with B2M. A 6X His-tag and sortase recognition motif (LPETG) have been installed at the C-terminus of each CH3 domain for purification and site-specific protein modification via sortase, respectively. B) Schematic of 64Cu-labeled synTac preparation for immunoPET imaging. Sortase was used to install the metal chelator G3-NOTA (NOTA: 1,4,7-triazacyclononane-N,N’,N”-triacetic acid), which is then radiolabeled with 64Cu prior to imaging. C) Coomassie stain (left) and western blot (right) of the HPV E7 H-2Db synTac before and after conjugation with G3-biotin via sortase. The samples were separated via SDS-PAGE under reducing conditions and stained with Coomassie reagent (left) or transferred to a PVDF membrane and probed with streptavidin-HRP (right). The synTac heavy chain runs at ~75 kDa when the disulfide bonds between the CH2 domains are reduced. The western blot shows that biotin was successfully installed on the synTac as only the sortase-modified sample results in luminescence when incubated with streptavidin-HRP and an HRP substrate. Shown is a representative example of an experiment performed three times.