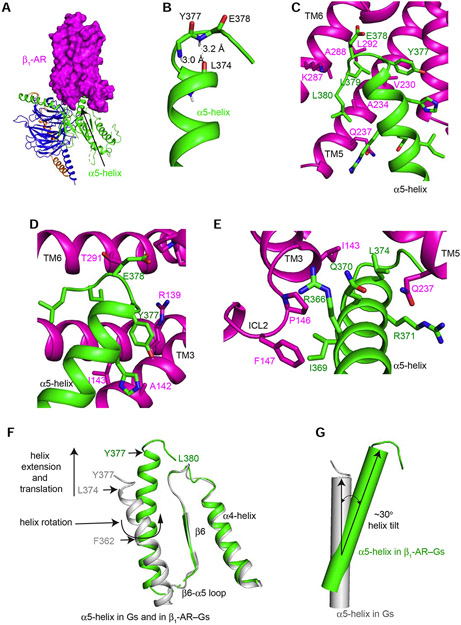
Figure 3. Structural rearrangements of the α5-helix of Gαs upon binding of β1-AR.
(A) β1-AR uses its cytoplasmic surface like a saddle to cradle the C-terminal α5-helix of the Ras-like domain of Gαs. (B) The last 4 amino acids (Tyr377 to Leu380) of α5-helix form a C-terminal αL capping motif with intra-chain interactions. (C and D) Interactions between β1-AR and the C-terminal tail loop of the α5-helix of Gαs. (E) Interactions between the middle of the α5-helix of Gαs and β1-AR. (F) Structural comparison of the α5-helix of Gαs from the β1-AR–Gs complex (colored in green) and from Gαs-GTPγS (colored in gray). (G) Tilting of the α5-helix of Gαs from Gαs-GTPγS (colored in gray) to the β1-AR–Gs complex (colored in green).