Abstract
Aliphatic α,ω‐dicarboxylic acids (DCAs) are a class of useful chemicals that are currently produced by energy-intensive, multistage chemical oxidations that are hazardous to the environment. Therefore, the development of environmentally friendly, safe, neutral routes to DCAs is important. We report an in vivo artificially designed biocatalytic cascade process for biotransformation of cycloalkanes to DCAs. To reduce protein expression burden and redox constraints caused by multi-enzyme expression in a single microbe, the biocatalytic pathway is divided into three basic Escherichia coli cell modules. The modules possess either redox-neutral or redox-regeneration systems and are combined to form E. coli consortia for use in biotransformations. The designed consortia of E. coli containing the modules efficiently convert cycloalkanes or cycloalkanols to DCAs without addition of exogenous coenzymes. Thus, this developed biocatalytic process provides a promising alternative to the current industrial process for manufacturing DCAs.
Subject terms: Biocatalysis, Enzymes, Biosynthesis
Aliphatic α,ω-dicarboxylic acids (DCAs) are widely used chemicals that are synthesised by multistage chemical oxidations. Here, the authors report an artificially designed biocatalytic cascade for the oxidation of cycloalkanes or cycloalkanols to DCAs in the form of microbial consortia, composed of three Escherichia coli cell modules.
Introduction
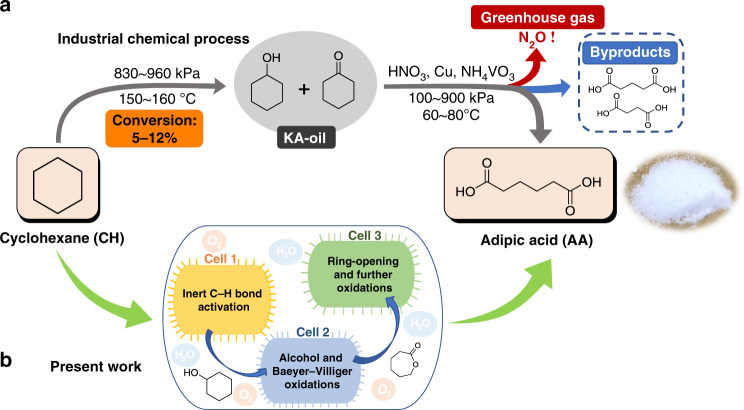
Aliphatic α,ω‐dicarboxylic acids (DCAs) are a class of chemicals extensively used in the preparation of perfumes, polymers, adhesives, and macrolide antibiotics1,2. Currently, the majority of industrial DCAs are synthesized by energy-intensive, hazardous multistage oxidations. For example, adipic acid (AA) is the most industrially important DCA, with a global market of about $6.3 billion per year3. The current industrial production process for AA relies on a two-step chemical oxidation under harsh conditions using cyclohexane (CH) as starting material (Fig. 1a)4. This industrial process has low efficiency with formation of succinic acid and glutaric acid as byproducts5. In addition, this process generates almost 10% of global anthropogenic nitrous oxide N2O emissions because it uses a large amount of nitric acid6. These emissions cause serious environmental problems such as global warming and ozone depletion. The alternative routes of directed oxidation of CH to AA with hydrogen peroxide7 and carbonylation of 1,3-butadiene to adipate diester by a designed palladium catalyst8 produce no nitrous oxide waste and are more environmentally friendly. However, substrate prices and technical challenges (such as catalyst stability, preparation of the catalyst, and heavy metal recovery) limit their implementation9.
Fig. 1. Industrial chemical and designed biocatalytic processes for adipic acid (AA) production.
a Current industrial process for synthesis of AA by multistage chemical oxidation from cyclohexane (CH). b designed one-pot biocatalytic route for synthesis of AA from CH using an Escherichia coli consortium, composed of three E. coli cell modules.
To address these challenges, environmentally friendly, biobased approaches for DCA production have been recently developed3. Biobased routes from renewable feedstocks such as glucose10 have been attempted. However, these routes require complex engineering of production microbes by metabolic engineering and synthetic biology techniques, and many challenges remain including redox constraints, enzyme optimization, selection of suitable production hosts and metabolic pathways, and potential harm to cell growth11–13. In addition, these methods generate only a limited range of DCA products.
Artificially designed multi-enzyme cascades are a useful tool for accomplishing challenging reactions that cannot be achieved by one-pot chemical catalysis14. Among them, the construction of an in vivo multi-enzymatic cascade offers many advantages over in vitro approaches since the costly steps (e.g., enzyme purification, addition of expensive cofactors) can be avoided15. For these reasons, the de novo design of in vivo cascade reactions has gained attention16–22 with successful examples such as the oxy- and amino-functionalization of alkenes20 and synthesis of α-functionalized organic acids from glycine and aldehydes17.
Nevertheless, protein expression burden and redox balance issues often arise when attempting to express multiple enzymes in a single microbe for the construction of in vivo whole-cell catalysts23. To solve these problems, and inspired by the metabolic engineering concept of using microbial consortia through co-culture of engineered organisms to improve production of targeted compounds23–26, we envisioned the design of microbial-consortia-mediated in vivo biocatalytic cascades with these following advantages: (i) protein expression burden and redox constraints can be reduced by distributing the biocatalytic pathway among different cell modules; (ii) each expression system or cell module can be constructed and optimized in parallel, substantially reducing development time; and (iii) the catalyst loading of each cell module can be adjusted, allowing beneficial interactions among cell modules to enhance productivity. In addition, to eliminate redox constraints and cross-contamination, cofactor self-sufficiency-based modularization can be employed to ensure that each cell module is either redox neutral or coupled to redox regeneration27,28. This strategy of modularization is rarely used.
Here, we develop an in vivo, artificially designed biocatalytic cascade for the oxidation of cycloalkanes or cycloalkanols to DCAs using a designed biocatalytic Escherichia coli consortia system modularized for redox self-sufficiency. With the oxidation of CH to AA as a model reaction, each basic cell module with assigned functions are engineered and optimized in parallel, followed by combinatorial optimization to achieve efficient production of AA from CH (Fig. 1b). Finally, the substrates are expanded to cycloalkanes with different carbon numbers to demonstrate the generality of the developed biocatalytic system.
Results
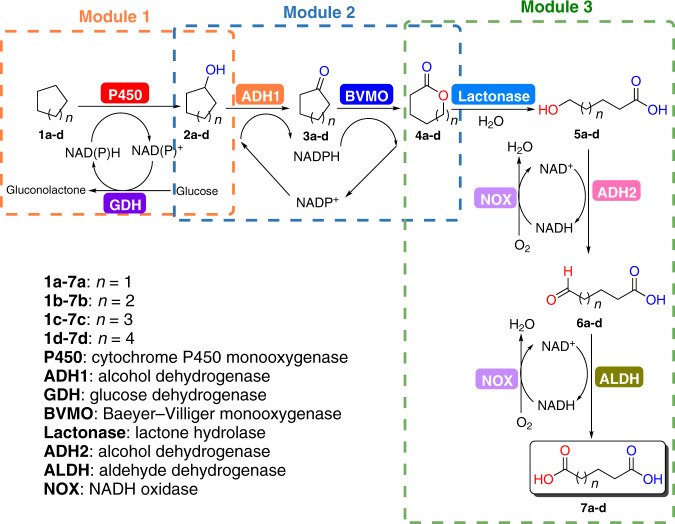
Design and modularization of a biocatalytic cascade
To implement the targeted production of different DCAs 7a-d from cycloalkanes 1a-d, we designed an artificial biosynthetic route based on biocatalytic retrosynthesis29 (Fig. 2). The cascade had six enzymatic reactions and eight enzymes (Fig. 2). The ideal in vivo cascade would be a single-cell biocatalyst harboring all necessary enzymes. Therefore, we first tried to construct E. coli cells expressing all enzymes needed to produce AA 7b from cyclohexanol (CHOL) 2b or CH 1b (Supplementary Figs. 1a, 2a). However, none showed satisfactory productivity with only trace amounts of product AA (2-4 mM) detected (Supplementary Figs. 1b, 2b). We hypothesized that this could be caused by expression burden from attempting to express multiple enzymes in a single E. coli cell, as well as redox imbalances from performing multiple redox-mediated oxidation reactions in the same reaction vessel (cell)28.
Fig. 2. Design and modularization of an artificial biocatalytic cascade.
Modularization was developed based on the rule that each module would be either redox neutral or coupled with a redox-regeneration system: Module 1 involves P450-catalyzed hydroxylation of cycloalkanes 1a-d coupled to a glucose dehydrogenase (GDH) mediated cofactor NAD(P)H regeneration. Module 2 comprises of a redox neutral system consisting of an alcohol dehydrogenase (ADH1) and a Baeyer-Villiger monooxygenase (BVMO), which catalyzes oxidation of cycloalkanols 2a-d to corresponding lactones 4a-d. Module 3 contains lactonase-catalyzed hydrolysis of lactones to hydroxyl acids 5a-d, followed by consecutive oxidations to DCA 7a-d with an alcohol dehydrogenase (ADH2) and an aldehyde dehydrogenase (ALDH), in which NAD+ regeneration was achieved in presence of NADH oxidase (NOX).
To address these issues, the biocatalytic cascade was divided into three modules based on the rule that each would be either redox neutral or coupled to redox regeneration (Fig. 2). Module 1 includes a P450-catalyzed hydroxylation of cycloalkanes 1a-d to corresponding alcohols 2a-d coupled to a glucose dehydrogenase (GDH) involved cofactor NAD(P)H regeneration. Module 2 is a redox neutral system30 that consists of an alcohol dehydrogenase (ADH1) catalyzes the oxidation of the alcohols 2a-d to ketones 3a-d, and a Baeyer-Villiger monooxygenase (BVMO) mediates the conversion of cycloalkanones to lactones 4a-d. Module 3 comprises the lactonase-catalyzed hydrolysis of lactones to hydroxyl acids 5a-d, and an alcohol dehydrogenase (ADH2) and an aldehyde dehydrogenase (ALDH) for consecutive oxidations to DCA products 7a-d with NADH oxidase (NOX)-mediated NAD+ regeneration. Each module was expressed in E. coli, resulting in three cell module catalysts designated as cell modules 1, 2, and 3. This potentially reduced the protein expression burden and avoided cofactor cross-contamination by confining the cofactor NAD(P)H/NAD(P)+ inside the cells.
As a first attempt, the constructed cell modules were combined (2 and 3; or 1, 2, and 3) without optimization to form an E. coli consortium (EC) for AA 7b production. Interestingly, AA 7b production increased by 9-fold (to 18 mM) and 6-fold (to 13 mM) when using, respectively, CHOL 2b and CH 1b as substrates (Supplementary Figs. 1b, 2b), supporting our hypothesis. Therefore, the EC system was further investigated systematically for DCA production from either cycloalkanols or cycloalkanes.
Engineering of basic cell module catalysts
The three basic cell module catalysts were constructed and optimized in parallel, then combined to form an EC for use in the conversion of cycloalkanes or cycloalkanols to DCAs. As a proof of concept, we used transformation of CHOL 2b or CH 1b to AA 7b as a model reaction.
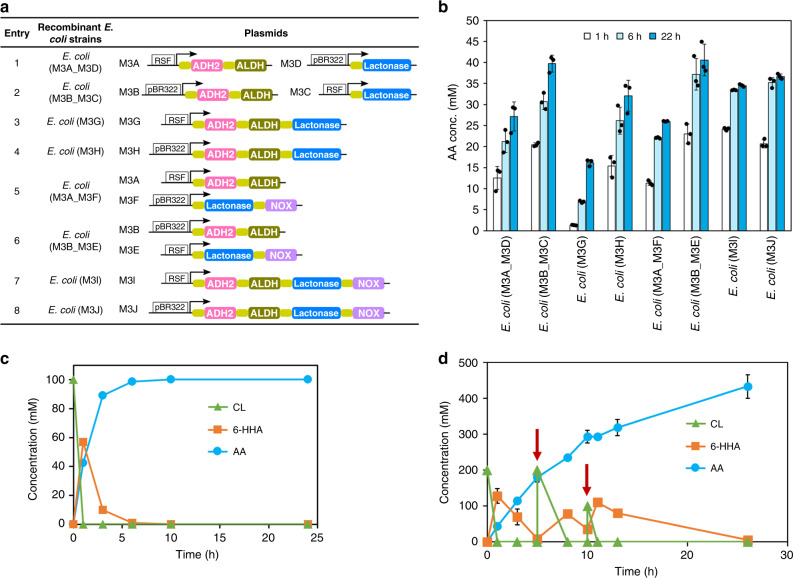
Engineering modular cell 3, responsible for conversion of ε-caprolactone (CL) 4b to AA 7b, we used lactonase from Rhodococcus sp. HI-3131 and alcohol dehydrogenase (ADH2) and aldehyde dehydrogenase (ALDH) from Acinetobacter sp. NCIMB987132,33. To provide sufficient NAD+ cofactor, NADH oxidase (NOX) originating from Lactobacillus brevis DSM 20054 was employed34. As shown in Fig. 3a, module 3 cells were constructed as different plasmid configurations, for a total of eight recombinant E. coli cells. These cells were individually examined and compared as catalysts for the conversion of 50 mM CL 4b to AA 7b (Fig. 3b). All cells produced AA 7b (16–42 mM), with E. coli (M3B_M3E) exhibiting the highest productivity of 42 mM AA 7b (84% yield) in 22 h with little intermediate accumulation (Fig. 3b). Thus, this whole-cell biocatalyst was chosen for subsequent applications. The reaction process of this biotransformation was monitored using 100 mM CL 4b (Fig. 3c). Within 2 h, the substrate was hydrolyzed to 6-hydroxyhexanoic acid (6-HHA) 5b, which was completely oxidized to AA 7b after 6 h. When the substrate concentration was increased to >100 mM, however, the intermediate 6-HHA 5b was not completely converted, even with prolonged reaction time. A possible reason for this was the reduced pH caused by AA in the reaction solution. Therefore, to further improve productivity, the reaction pH was maintained by adjustment and substrate was added in fed-batch mode. As shown in Fig. 3d, a total of 500 mM substrate 4b was added in three portions at predetermined times. Product AA 7b amounted to 433 mM (87% yield) within 26 h with only 5 mM 6-HHA 5b intermediate. From a practical viewpoint, the 1% side product was negligible.
Fig. 3. Engineering cell module 3 for conversion of CL 4b to AA 7b.
a Construction of E. coli cells expressing lactonase, alcohol dehydrogenase (ADH2), aldehyde dehydrogenase (ALDH) and NADH oxidase (NOX). See Supplementary Fig. 3 for SDS-PAGE of whole-cell proteins of Module 3 expressed in E. coli. RSF: pRSFDuet-1; pBR322: pETDuet-1; arrow: T7 promoter. Pink filled rectangle: ADH2 gene; green filled rectangle: ALDH; blue filled rectangle: lactonase gene; purple filled rectangle: NOX gene. b Engineered E. coli cells containing enzyme module 3 (lactonase, ADH2 and ALDH, and NOX if necessary) for biotransformation of CL 4b to AA 7b. Blue column: AA. c Time course of E. coli (M3B_M3E)-catalyzed transformation of CL 4b to AA 7b at substrate concentration 100 mM. Blue line: AA; orange line: 6-HHA; green line: CL. d Time course of E. coli (M3B_M3E)-catalyzed transformation of CL 4b to AA 7b, with substrate added in three portions (200 mM in the beginning, and 200 mM and 100 mM indicated by arrows) for a total concentration of 500 mM with pH maintained by adjustment. Blue line: AA; orange line: 6-HHA; green line: CL. The data shown in b, c, and d are presented as mean value ± SD (standard deviations) of three biological replicates. Source data are provided as a Source Data file.
In a previous study35, a cascade module was designed to convert CL 4b to 6-aminohexanoic acid. However, the dead-end intermediate 6-HHA 5b was formed, which cannot be oxidized by the alcohol dehydrogenases tested (prim-ADH from Bacillus stearothermophilus). Instead, the esterase 008-SD from Bacillus subtilis was used to catalyze methyl esterification of the precursor ε-caprolactone in the presence of methanol. In contrast, ADH2 from Acinetobacter sp. NCIMB9871 used in our system accepts 6-HHA 5b as a good substrate, preventing the dead-end. In addition, a recent report indicates that CL 4b can be produced from biobased fructose36. Thus, our strains could also provide a route (Supplementary Fig. 4) for biobased renewable production of AA 7b at ca. 66 g L−1, comparable to the highest value (68 g L−1) reported using engineered E. coli cells with glucose as a carbon source13.
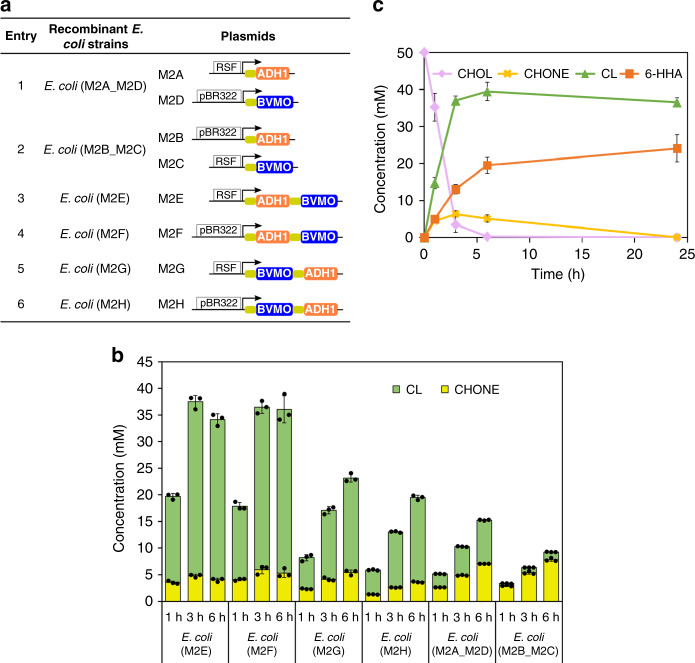
To engineer cell module 2 for converting CHOL 2b to CL 4b, we used an alcohol dehydrogenase (ADH1) from Lactobacillus brevis ATCC 1486937 and a Baeyer-Villiger monooxygenase (BVMO) originating from Acinetobacter sp. NCIMB9871 with two mutations (C376L/M400I)38 conferring higher oxidative stability compared to the wild-type. The two enzymes formed a redox-neutral system in which NADPH was recycled by a hydrogen-borrowing process30. Similarly, six recombinant E. coli host cells were obtained with different combinations of ADH1 and BVMO (Fig. 4a). The resulting E. coli strains were tested for conversion of 50 mM CHOL 2b to CL 4b with cyclohexanone (CHONE) 3b as an intermediate (Fig. 4b). Cells with one-plasmid systems showed much higher productivity than cells with two-plasmid systems. Among one-plasmid systems, E. coli (M2E) had the highest catalytic performance, producing 32 mM CL 4b (64% yield) in 3 h. We found that product concentration was lower than expected due to autohydrolysis of the product CL 4b to 6-HHA 5b in the reaction buffer system. The whole-cell catalyst E. coli (M2E) was chosen for subsequent reactions.
Fig. 4. Engineering of cell module 2 for conversion of CHOL 2b to CL 4b.
a Construction of E. coli cells expressing alcohol dehydrogenase (ADH1) and Baeyer-Villiger monooxygenase with double mutation. See Supplementary Fig. 5 for SDS-PAGE of whole-cell proteins of module 2 expressed in E. coli. RSF: pRSFDuet-1; pBR322: pETDuet-1; arrow: T7 promoter. Orange filled rectangle: ADH1 gene; blue filled rectangle: BVMO gene. b Engineered E. coli cells containing enzymes of module 2 (ADH1 and BVMO) for the biotransformation of CHOL 2b to CL 4b. Green column: CL; yellow column: CHONE. c Time course of E. coli (M2E)-catalyzed transformation of CHOL 2b to CL 4b at substrate concentration 50 mM. Orange line: 6-HHA; green line: CL; yellow line: CHONE; purple line: CHOL. The data shown in b and c are presented as mean value ± SD (standard deviations) of three biological replicates. Source data are provided as a Source Data file.
For the construction of cell module 2, we attempted to express both enzymes (BVMO and ADH1) from the same T7 promoter in one plasmid with a ribosome binding site (RBS) in between. A more balanced, strong protein expression was achieved when the ADH1 gene was placed next to the T7 promoter. Using the best cell module 2 as catalyst, as much as 50 mM CHOL 2b was converted to products without addition of exogenous cofactors and cosubstrates, which was much higher than production from an E. coli cell catalyst (20 mM) expressing both enzymes with two individual T7 promoters in the presence of glucose and acetone as cosubstrates39.
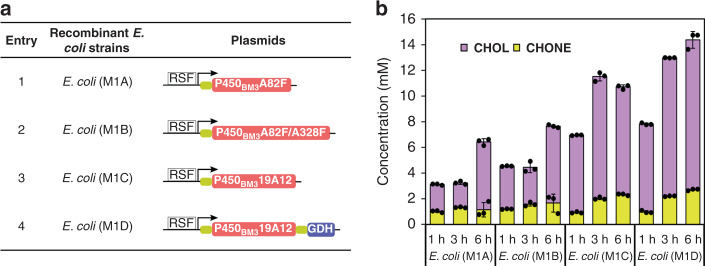
The activation of an inert C-H bond of cycloalkanes for formation of the corresponding alcohol is challenging. Thus far, only a few reports have used P450 monooxygenase for the hydroxylation of CH 1b to CHOL 2b. A common issue is low activity40–43. In this study, the self-sufficient cytochrome P450BM3, a long-chain fatty acids monooxygenase from Bacillus megaterium, was considered. It exhibits the highest reported monooxygenase activity among P450 enzymes towards its natural substrates. However, the activity is essentially lost for small molecules such as propane, cyclohexane or benzene. In our earlier study, P450BM3 mutants were generated with high activity towards structurally small substrates (CH 1b and CHONE 3b) by introducing the relatively large hydrophobic phenylalanine to reduce the size of the binding pocket16,19. The two most active mutants, P450BM3 A82F and A82F/A328F were employed in cell module 1. In addition, the previously engineered mutant P450BM3 19A12, which contains 20 mutations18 and has relatively high activity for CH 1b, was also tested and compared for biotransformation of CH 1b to CHOL 2b. As shown in Fig. 5, three recombinant E. coli cells expressing three different P450BM3 variants were tested for transformation at a CH concentration of 50 mM (Fig. 5b). E. coli (M1C) containing P450BM3 mutant 19A12 showed the highest activity, producing ca. 9.5 mM CHOL 2b within 3 h. This result was much higher than with the other P450s (CYP_CHX and CYP153A6), which catalyzed the same reactions and had a product concentration ranging from 10 μM to 4 mM41. Subsequently, a cofactor regeneration system was constructed by introducing GDH based on P450BM3 mutant 19A12 (Fig. 5a, Entry 4), which further improved the productivity to 11.5 mM CHOL 2b and 2.5 mM CHONE 3b. The resulting E. coli (M1D) as cell module 1 was employed in cascade reactions for AA production. However, cell module 1 was still less efficient than the constructed cell modules 2 and 3. We surmised that the problem would be solved to a certain extent after coupling to the cell modules 2 and 3 because the constant removal of CHOL would reduce product inhibition.
Fig. 5. Engineering of cell module 1 for converting CH 1b to CHOL 2b.
a Construction of E. coli cells expressing P450BM3 variants (A82F, A82F/A328F and 19A12) and glucose dehydrogenase (GDH). See Supplementary Fig. 6 for SDS-PAGE of whole-cell proteins of module 1 expressed in E. coli. RSF: pRSFDuet-1; arrow: T7 promoter. Red filled rectangle: P450BM3 variants genes; blue filled rectangle: GDH gene. b Engineered E. coli cells containing enzyme module 1 (P450BM3 variants) for the biotransformation of CH 1b to CHOL 2b. Purple column: CHOL; yellow column: CHONE. The data shown in b are presented as mean value ± SD (standard deviations) of three biological replicates. Source data are provided as a Source Data file.
Construction of E. coli consortia for AA 7b production
After generating the three selected cell modules, western blot was carried out to examine the enzyme expression of cells containing the modules: E. coli (M3B_M3E), E. coli (M2E) and E. coli (M1D). For comparison, the protein expression of all needed enzymes (modules 1 + 2 + 3 or modules 2 + 3) in a single E. coli cell was also conducted. Five of eight enzymes (P450, ADH1, ADH2, ALDH and lactonase) were expressed with His-tag, while the others (GDH, BVMO and NOX) were expressed with Flag-tag (Supplementary Table 1). A general trend can be found that the expressions of almost all the recombinant enzymes in individual module system were comparable or higher than those in single E. coli strain composed of multiple modules. For instance, the expression levels of GDH in cell module 1, ADH1 and BVMO in cell module 2 were much higher compared to cells containing multiple modules (Supplementary Fig. 7). P450 was detectable in the cell module 1, but not in cell expressing enzymes modules 1, 2, and 3. Meanwhile, we also determined P450 concentrations based on CO-binding difference spectra44. The result showed that P450 concentration in cell module 1 was 2.20 μM, but P450 was undetectable in the cell containing enzyme modules 1, 2, and 3 (Supplementary Table 2), which is in accordance with the western blot results. Therefore, we expected much higher productivity with the E. coli consortia of a combination of cell modules.
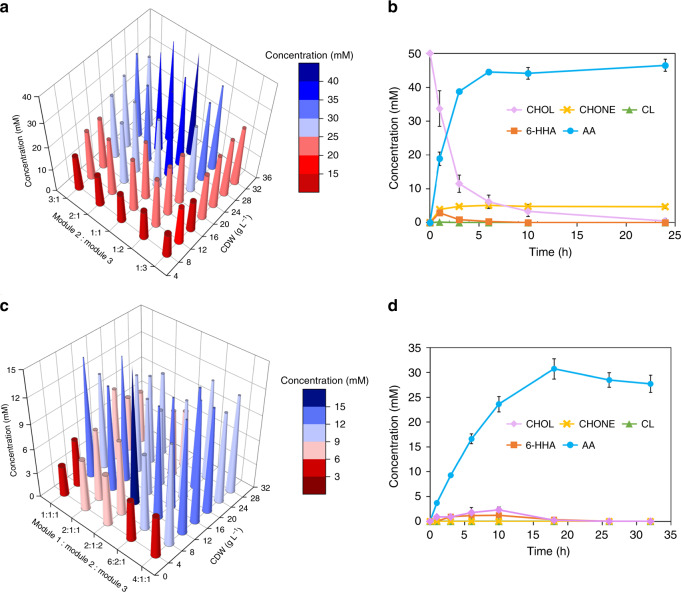
Next, we tested the one-pot conversion of CHOL 2b to AA 7b with the E. coli consortium 2_3 (EC2_3) composed of selected cell modules 2 and 3. Optimization of the EC2_3-catalyzed one-pot conversion was conducted by adjusting the ratio and total amount (cell density) of the two modules in the consortium. As shown in Fig. 6a, AA 7b formation was detected under different conditions. Product concentration increased with increased catalyst loading (8–32 g cell dry weight [CDW] L−1) and a 1:1 ratio of cell catalysts showed the highest catalytic performance. Conversion of CHOL 2b to AA 7b was investigated with this E. coli consortium of modules 2 and 3 under optimized conditions. CHOL 2b was converted to provide 46 mM AA 7b after 24 h reaction (Fig. 6b), which was more than 10-fold higher than production by a single strain containing modules 2 and 3 (2–3 mM AA). Although some examples exist of in vitro biocatalytic cascades starting from CHOL 2b and ending with 6-aminohexanoic acid and oligomers of ε-caprolactone as products in the presence of expensive cofactors and cosubstrates35,43, an artificially designed in vivo biocatalytic route to AA 7b from CHOL 2b has not been reported. CHOL-degrading bacteria such as Arthrobacter sp. and Rhodococcus sp. have been found that naturally degrade the cyclohexanol via a similar route, but they further metabolized AA and thus it does not accumulate33.
Fig. 6. Construction of E. coli consortia for the production of AA 7b.
a Optimization of conditions for E. coli consortium 2_3 (EC2_3)-catalyzed conversion of CHOL 2b to AA 7b with a reaction time of 6 h. b Time course for EC2_3 catalyzed conversion of CHOL 2b to AA 7b under optimized conditions at 50 mM substrate. Blue line: AA; Orange line: 6-HHA; green line: CL; yellow line: CHONE; purple line: CHOL. c Optimization of conditions for E. coli consortium 1_2_3 (EC1_2_3)-catalyzed conversion of CH 1b to AA 7b with a reaction time of 6 h. d Time course for EC1_2_3-catalyzed conversion of CH 1b to AA 7b under optimized conditions at 100 mM substrate. Blue line: AA; Orange line: 6-HHA; green line: CL; yellow line: CHONE; purple line: CHOL. The data shown in a and c are presented as mean value of two biological replicates. The data shown in b and d are presented as mean value ± SD (standard deviations) of three biological replicates. Source data are provided as a Source Data file.
To examine the scalability of the developed E. coli consortia system EC2_3 for AA 7b production from CHOL 2b, the reaction was conducted in 1-L fermenter with 400 mL reaction mixture containing 50 mM CHOL 2b. Without further optimizations, 44 mM of AA 7b was produced after 36 h reaction (Supplementary Figs. 8, 9), which was comparable to that obtained in shaking flasks (Fig. 6b).
Finally, the cell modules 1, 2 and 3 with optimal functions were combined to form the E. coli consortium 1_2_3 (EC1_2_3) for the conversion of CH 1b to CHOL 7b. The reaction conditions including the ratios of modular cells and cell loading were likewise investigated. As shown in Fig. 6c, the highest product concentration was obtained at a cell loading of 12 g CDW L−1, which then decreased when the cell density was above it. A possible reason for this could be sensitivity of P450 to poor oxygen transfer and limited hydrophobic substrate availability caused by increased viscosity at high cell density, leading to reduced catalytic efficiency. This hypothesis needs to be addressed in a further study. Optimization of the ratio of whole-cell catalysts showed that 2:1:2 (modules 1:2:3) had the best catalytic performance. Consortium EC1_2_3 catalyzed the conversion of CH 1b to AA 7b under optimized conditions with 100 mM substrate, reaching a maximum of 31 mM AA 7b in 20 h without intermediate accumulation (Fig. 6d), which was about 10-fold higher than production by a single strain containing modules 1, 2 and 3 (3-4 mM AA) (Supplementary Fig. 2b). This maximum was also more than 2-fold higher than when module 1 was used alone (Supplementary Fig. 10), confirming our hypothesis that coupling the enzymatic reactions alleviated the product inhibition. In addition, no metabolism of substrate, intermediates or product by host E. coli cells was observed during the cascade reactions.
Considering the importance of the stability of the developed E. coli consortia, we determined the catalytic performance of each cell module after preincubation under reaction conditions. It was shown that both cell module 1 and 3 retained ~81% of their catalytic ability, while the percentage for module 2 was significantly reduced to only 47% after 24 h preincubation (Supplementary Fig. 11), suggesting the poor stability of module 2. In addition, the viability of E. coli cells during the EC1_2_3-catalyzed reaction was tested using the LIVE/DEAD® BacLight™ Bacterial Viability kit. The results showed that the percentage of viable cells with undamaged membrane dropped to ~50% just after adding the substrate CH 1b, then further reduced to only 13% at 3 h reaction (Supplementary Fig. 12). The rapid reduction of live cell percentage might be due to the membrane damage caused by the strong hydrophobic nature of substrate CH 1b. However, we would like to stress that, the non-viable cells may still have the desired enzymatic activities, leading to the difficulty in the accurate measurement of cells with enzymatic activities especially in a continuous and dynamic manner. Furthermore, the increased membrane permeability of E. coli cells may benefit the access of substrate and product molecules, accelerating the microbial consortia-based cascade reactions45.
The aforementioned difference in stability of each cell module and reduced viability of cells during the reactions could be addressed by some solutions46,47: (a) intermittent supplementation of underdog subpopulations to elongate the modular reaction; (b) cell immobilization and enzyme engineering to improve the robustness of cell catalysts; (d) establishment of the biocompatible biphasic system (e.g. ionic-water or organic-water system) with the substrate deposited in ionic or organic phase, to prevent the cells in the aqueous phase from being damaged by the hydrophobic CH 1b.
Next, the scalability of the developed E. coli consortia EC1_2_3 was likewise examined for converting CH 1b in 1-L fermenters with 400 mL reaction mixture. A ratio of 2:1:2 (final CDW was 12 g L−1, module 1: module 2: module 3) was first tested for conversion of 200 mM CH 1b. The reaction system still works but with low efficiency, the titer of AA 7b after 36 h reaction was around 4 mM (Supplementary Fig. 13a). The possible reasons could be the different conditions (e.g., agitation and oxygen mass transfer) between shaking flasks and fermenters, which still needs further study. Considering the successful scaled-up of CHOL 2b to AA 7b with EC2_3 (Supplementary Fig. 8), a two-step addition of catalysts strategy was attempted, in which cell module 1 was first used to convert CH 1b to CHOL 2b with a certain accumulation, followed by addition of module 2 and module 3 to convert CHOL 2b to AA 7b. Consequently, AA 7b production was boosted to 22 mM after 36 h reaction (Supplementary Fig. 13b), demonstrating the potential of scalability of the developed E. coli consortia system as well as its advantages due to the flexible operational mode for improving the productivity.
Substrate scope of the developed E. coli consortia system
To determine the substrate scope and generality of the E. coli consortia system, different cycloalkanols 2a-d and cycloalkanes 1a-d were tested (Table 1). For all substrates tested using EC2_3 as catalyst, very high conversion of cycloalkanols to corresponding DCAs were obtained. Product concentrations were high (42–49 mM) with limited accumulation of intermediates as undesired side products. For the EC1_2_3-catalyzed conversion of cycloalkanes to corresponding DCAs, somewhat lower product concentrations (6–21 mM) were achieved, possibly due to low catalytic efficiency in the first step of the P450-catalyzed reaction. Finally, preparations of the four DCAs from cycloalkanes were conducted with a simple workup procedure of extraction for unreacted substrate recovery followed by adjustment to pH 1–2 and subsequent extraction to obtain pure product (see the Experimental Section for details), which led to isolated product yields of 13–45%. This demonstrated the generality of the E. coli consortia biocatalytic system.
Table 1.
One-pot DCAs production with designed E. coli consortia.
| Entry | Substrates | Conc. of DCAsa (mM) | Product distribution [%]b | ||||
|---|---|---|---|---|---|---|---|
| 2a-d | 3a-d | 4a-d | 5a-d | 7a-d | |||
| 1c | n = 1, 2a | 48 | 0 | 7 | 0 | 0 | 93 |
| 2c | n = 2, 2b | 46 | 0 | 9 | 0 | 0 | 91 |
| 3c | n = 3, 2c | 49 | 0 | 0 | 0 | 0 | >99 |
| 4c | n = 4, 2d | 42 | 0 | 0 | 0 | 7 | 93 |
| 5d | n = 1, 1a | 12 | 0 | 0 | 0 | 0 | >99 |
| 6d | n = 2, 1b | 21 | 0 | 0 | 0 | 0 | >99 |
| 7d | n = 3, 1c | 20 | 0 | 0 | 0 | 0 | >99 |
| 8d | n = 4, 1d | 6.1 | 17 | 13 | 0 | 17 | 53 |
aDetermined by gas chromatography.
bRelative amounts based on the concentrations of dicarboxylic acids 7a-d and analyzed by gas chromatography. Data are presented as mean value of two biological replicates. Source data are provided as a Source Data file.
cReactions were conducted with indicated substrates (50 mM) in 4 mL cell suspensions at 16 g CDW L−1. EC2_3 containing E. coli (M2E) and E. coli (M3B_M3E) at a ratio of 2:1 was in 200 mM KP buffer (pH 8.0) at 25 °C and 200 rpm for 24 h.
dReactions were conducted with indicated substrates (50 mM) in 4 mL cell suspension at 12 g CDW L−1. EC1_2_3 containing E. coli (M1E), E. coli (M2E), and E. coli (M3B_M3E) at ratio 2:1:2 was in 200 mM KP buffer (pH 8.0) with 0.05 g mL−1 glucose at 25 °C and 200 rpm for 24 h.
Compared with other biobased fermentation methods with engineered E. coli for DCAs production48, our biocatalytic route provides general access to DCAs with varying chain length (C5 to C8) from different starting chemicals (e.g., cycloalkanes, cycloalkanols, or lactones). In contrast, metabolic engineering strategies require individual engineering of metabolic pathways for each DCA product48. In addition, with cycloalkanes or cycloalkanols as substrates, our approach gave much higher product titers than fermentation methods for production of glutaric acid 7a (1.6–6.3 g L−1 vs. 0.82 g L−1)49 and suberic acid 7d (1.1–7.3 g L−1 vs. 0.254 g L−1)50, and for pimelic acid 7c, which has not been realized by metabolic pathway construction in E. coli. For AA 7b, product titer was as high as 66 g L−1 using biobased CL as substrate, which is comparable to the highest value reported (68 g L−1)13. For downstream processing, our biocatalytic process offers an easier product purification procedure because it uses resting cells as catalysts in a buffered system. In fermentations with engineered host cells, costly, complicated multistep processes (e.g., extraction, chromatography and recrystallization) are often required because of impurities in the culture medium and metabolites or byproducts from growing cells. A comparison of our approach to other biobased methods is summarized in Supplementary Table 3.
In summary, the biocatalytic process we developed is a general approach for one-pot synthesis of DCAs using either cycloalkanes or cycloalkanols as starting materials. This process is an ideal solution to the problems encountered in the industrial chemical processes. The easier product isolation and substrate recovery procedure of our biocatalytic process shows great advantages over chemical and fermentation methods. The concept of microbial-consortia-mediated biocatalytic pathway reconstruction and redox self-sufficiency-based modularization provides solutions and guidance for further development of in vivo artificial biocatalytic cascades for challenging transformations. To further improve the efficiency of this biocatalytic system, future work will focus on engineering rate-limiting enzymes such as P450 and BVMO, fine-tuning protein expression by promoter and RBS engineering, and scale-up of the bioprocess in bioreactors with precisely controlled parameters.
Methods
Construction of recombinant E. coli
DNA fragments of genes encoding enzymes and the linear plasmid backbone were amplified by PCR using primers with 15- to 20-bp homologous arms that enabled the subsequent recombination. Genes for enzymes were assembled via overlap PCR and cloned into linear vector in the presence of T5 exonuclease to generate 15-bp or 20-bp sticky ends to promote recombination efficiency. Reaction mixtures were 5 µL containing linear vector, enzyme genes, buffer 4.0 (New England Biolabs) and T5 exonuclease, incubated in ice-water for 5 min, followed by quick addition of 50 µL competent cells (E. coli DH5α) for transformation and plating on LB agar containing appropriate antibiotics. Resulting transformants were picked and DNA sequenced for confirmation. Plasmids containing targeted enzyme genes were transformed into E. coli BL21 cells for protein expression and whole-cell biocatalyst preparation. The detailed information of the strain and plasmids, primers and synthetic gene sequences was listed in Supplementary Tables 4–6.
Protein expression and preparation of cell module biocatalysts
Constructed E. coli cells were inoculated into 3 mL LB medium containing antibiotics (50 μg mL−1 kanamycin, 100 μg mL−1 ampicillin or both), and cultured at 37 °C, 220 rpm for 6 h. Precultures (1 mL) were transferred into 50 mL TB medium with appropriate antibiotics in 250-mL shaking flasks and cultured at 37 °C, 220 rpm for 2–3 h until OD600 0.6–0.8, then IPTG was added to a final concentration of 0.2 mM. The temperature was shifted to 25 °C for 14–16 h. For E. coli cells containing enzyme module 3, protein expression conditions were modified to: (a) 0.1 mM IPTG, and (2) protein expression at 20 °C for 20 h after IPTG addition. Cells were harvested by centrifugation at 3040 × g, 15 °C for 10 min, washed with 200 mM potassium phosphate buffer (pH 8.0) and used as whole-cell biocatalysts.
Typical procedure for cell module 3 converting CL 4b to AA 7b
The substrate CL 4b was added to a 4-mL suspension of E. coli cells expressing module 3 (final CDW was 16 g L−1) in potassium phosphate buffer (0.2 M, pH 8.0). Reactions were at 30 °C, 200 rpm in 100 mL shaking flasks with screw caps with substrate addition in fed-batch mode (200 mM 4b, followed by 200 mM and 100 mM 4b after 6 h and 10 h). The pH was maintained around 8.0 by adding 10 M NaOH. Samples were taken at appropriate intervals and prepared for GC analysis. Sample preparation for GC to determine AA 7b and 6-HHA 5b was 450 µL water, 50 µL HCl (4 M) and 500 µL ethyl acetate (EtOAc) added to each 50-µL reaction sample with vortexing and centrifuging (13,680 × g, 1 min). The organic phase was collected and dried over anhydrous Na2SO4 for derivatization and GC analysis with a SH-Rtx-1 column. To determine CL 4b, reaction samples were prepared for GC analysis with a SH-Rtx-WAX column by adding 450 µL water and 500 µL EtOAc containing 2 mM n-decane (internal standard) to 50 µL reaction sample, followed by vortexing and centrifuging (13,680 × g, 1 min). The organic phase was dried over anhydrous Na2SO4 and directly used for gas chromatography (GC). The result of cell module 3 catalyzed CL 4b to AA 7b at varying substrate concentrations is presented in Supplementary Fig. 14a.
Typical procedure for cell module 2 converting CHOL 2b to CL 4b
For substrate, 21.5 µL CHOL (2b, final concentration 50 mM) was added to a 4-mL suspension of modular E. coli cells expressing enzymes of module 2 (final CDW was 8 g L−1) in potassium phosphate buffer (0.1 M, pH 8.0). Reactions were at 25 °C, 200 rpm in 100-mL shaking flasks with screw caps. Samples were taken at appropriate intervals and prepared for GC analysis. Typically, to determine 6-HHA 5b, 400 µL water, 50 µL HCl (4 M) and 500 µL EtOAc were added to 100 µL reaction sample. Mixtures were vortexed and centrifuged (13,680 × g, 1 min). The organic phase was dried over anhydrous Na2SO4 for derivatization and GC analysis with a SH-Rtx-1 column. To determine CHOL 2b, CHONE 3b and CL 4b, reaction samples were prepared for GC analysis with a SH-Rtx-WAX column by adding 400 µL water and 500 µL EtOAc containing 2 mM n-decane (internal standard) to 100 µL reaction sample, followed by vortexing and centrifuging (13,680 × g, 1 min). The organic phase was dried over anhydrous Na2SO4 and used directly for GC analysis. The result of cell module 2 catalyzed CHOL 2b to CL 4b at varying substrate concentrations is presented in Supplementary Fig. 14b.
Typical procedure for cell module 1 converting CH 1b to CHOL 2b
The 22 µL substrate CH (1b, final concentration 50 mM) was added to a 4-mL suspension of modular E. coli cells expressing enzymes of module 1 (final CDW was 8 g L−1) in potassium phosphate buffer (0.1 M, pH 8.0) containing 0.05 g mL−1 glucose to facilitate NADPH regeneration. Reactions were at 25 °C, 200 rpm in 100-mL shaking flasks with screw caps. Samples were taken at appropriate intervals and prepared for GC analysis with a SH-Rtx-WAX column as described for cell module 2 converting 2b to 4b. The result of cell module 1 catalyzed CH 1b to CHOL 2b at varying substrate concentrations is presented in Supplementary Fig. 14c.
Typical procedure for E. coli consortium 2_3 converting 2a-d to 7a-d
The cycloalkanol (final concentration 50 mM; 18.3 µL cyclopentanol 2a, 21.5 µL cyclohexanol 2b, 24.8 µL cycloheptanol 2c, or 28.4 µL cyclooctanol 2d) was added to a 4-mL suspension of E. coli consortium 2_3 (final CDW was 16 g L−1, ratio of modular cell 2 and modular cell 3 was 2:1) in potassium phosphate buffer (0.2 M, pH 8.0). Reactions were at 25 °C, 200 rpm in 100-mL shaking flasks with screw caps. The pH was maintained around 8.0 by adding 10 M NaOH. Samples were taken at appropriate intervals and prepared for GC analysis as described for cell module 2 converting CHOL 2b to CL 4b (see GC chromatograms in Supplementary Figs. 15, 16).
Typical procedure for E. coli consortium 1_2_3 converting 1a-d to 7a-d
Cyclohexane 1b (44 µL, final concentration was 100 mM) was added to a 4-mL suspension of E. coli consortium 1_2_3 (final CDW was 12 g L−1, ratio of modular cells 1, 2 and 3 was 2:1:2) in potassium phosphate buffer (0.2 M, pH 8.0). We added 0.05 g mL−1 glucose initially to facilitate NADPH regeneration and 50 mM final substrate concentration (19.5 µL 1a, 22 µL 1b, 24.7 µL 1c, or 27.5 µL 1d) was used for substrate scope examination. Reactions were at 25 °C, 200 rpm in 100-mL shaking flasks. The pH was maintained around 8.0 by adding 10 M NaOH. Samples were taken at appropriate intervals and prepared for GC analysis as described for modular cell 2 converting 2b to 4b (see GC chromatograms in Supplementary Figs. 15, 17).
Preparative procedure for α, ω-dicarboxylic acids 7a-d
The cycloalkanes 1a-d (final concentration was 100 mM; 78 µL 1a, 86.8 µL 1b, 98.8 µL 1c, or 109.8 µL 1d) were added into an 8-mL suspension of E. coli consortium 1_2_3 (final CDW was 12 g L−1, ratio of modular cells 1, 2 and 3 was 2:1:2) in potassium phosphate buffer (0.2 M, pH 8.0) containing 0.05 g mL−1 glucose. Reactions were at 25 °C, 200 rpm in 250-mL shaking flasks for 24 h. For 1d, to ensure complete conversion of intermediate products 5d to corresponding α, ω-dicarboxylic acids 7d, a 2-mL suspension of E. coli cell module 3 (32 g CDW L−1) in potassium phosphate buffer (0.2 M, pH 8.0) was added after 24 h. During the reaction, the pH was maintained around 8.0 by adding 10 M NaOH. After reaction, mixtures were extracted three times with 30 mL EtOAc, and the organic phase was evaporated for substrate recovery. The water phase was acidified to below pH 2.0 with 4 M HCl, followed by three extractions with 50 mL EtOAc. The organic phase was dried over anhydrous Na2SO4. The solvent was removed using a rotary evaporator, and white solids were obtained at 13-45% isolated yields with purity >98% (glutaric acid 7a: 13.4 mg, yield = 13%; adipic acid 7b: 38.5 mg, yield = 33%; pimelic acid 7c: 57.8 mg, yield = 45%; octanedioic acid 7d: 18.8 mg, yield = 13%). Isolated products were subjected to GC-MS and NMR analysis (see chromatograms in Supplementary Figs. 18–21): 7a: 1H NMR (400 MHz, CD3OD): δ 2.35 (t, J = 7.4 Hz, 4H), 1.86 (p, J = 7.4 Hz, 2H); 7b: 1H NMR (400 MHz, CD3OD): δ 2.31 (ddt, J = 7.5, 5.7, 2.1 Hz, 4H), 1.68–1.59 (m, 4H); 7c: 1H NMR (400 MHz, CD3OD): δ 2.29 (t, J = 7.4 Hz, 4H), 1.62 (p, J = 7.5 Hz, 4H), 1.44–1.31 (m, 2H); and 7d: 1H NMR (400 MHz, CD3OD): δ 2.28 (t, J = 7.4 Hz, 4H), 1.72–1.52 (m, 4H), 1.36 (m, 4H).
Derivatization
To remove Na2SO4, obtained mixtures (products in EtOAc) were centrifugated at 13,680 × g for 10 min, and 300 µL supernatant solutions were transferred to fresh 1.5 mL tubes. After EtOAc evaporation, the resulting solid was dissolved in 30 µL N-methyl-N-(trimethylsilyl) trifluoroacetamide and 60 µL pyridine. Derivatization reactions were at 65 °C for 1 h and mixtures were used for GC analysis with an SH-Rtx-1 column.
GC analysis
For GC analysis with an SH-Rtx-1 column: 90 µL EtOAc containing an internal standard (25 mM n-decane) was added to derivative mixtures. Samples were analyzed using a SHIMADZU Nexis GC-2030 system equipped with a flame-ionization detector and SH-Rtx-1 column (30 m × 0.25 mm, 0.25 µm). Temperatures of injector and detector were 250 °C and 280 °C, respectively. Temperature program was: 5 °C per min from 50 °C to 120 °C, 40 °C per min to 240 °C, and held at 240 °C for 1 min.
For GC analysis with an SH-Rtx-WAX column (30 m × 0.25 mm, 0.25 µm): Obtained mixtures were analyzed with the temperature program: 5 °C per min from 50 °C to 120 °C, 40 °C per min to 240 °C, and held at 240 °C for 3 min.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2019YFA09005000), by the National Natural Science Foundation of China (No. 21977026 and 21702052) and Research Program of State Key Laboratory of Biocatalysis and Enzyme Engineering.
Source data
Author contributions
A.L. conceived and supervised the project, F.W., J.Z., and Q.L. performed the experiments and analyzed the data; J.Y., R.L., J.M., X.Y., G.W.Z., H.L.Y., C.Z., C.G.A., and L.M. analyzed the data; A.L. wrote the manuscript; all authors checked and modified the manuscript.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding author on reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Ying-Jin Yuan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fei Wang, Jing Zhao, Qian Li.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-18833-7.
References
- 1.Lee H, et al. Biotransformation of dicarboxylic acids from vegetable oil-derived sources: current methods and suggestions for improvement. Appl. Microbiol. Biotechol. 2019;103:1545–1555. doi: 10.1007/s00253-018-9571-7. [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta S, Hammond WB, Goddard WA. Crystal structures and properties of nylon polymers from theory. J. Am. Chem. Soc. 1996;118:12291–12301. doi: 10.1021/ja944125d. [DOI] [Google Scholar]
- 3.Deng Y, Ma LZ, Mao Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016;105:16–26. doi: 10.1016/j.bej.2015.08.015. [DOI] [Google Scholar]
- 4.Noack H, Georgiev V, Blomberg MRA, Siegbahn PEM, Johansson AJ. Theoretical insights into heme-catalyzed oxidation of cyclohexane to adipic acid. Inorg. Chem. 2011;50:1194–1202. doi: 10.1021/ic101405u. [DOI] [PubMed] [Google Scholar]
- 5.Castellan A, Bart JCJ, Cavallaro S. Industrial production and use of adipic acid. Catal. Today. 1991;9:237–254. doi: 10.1016/0920-5861(91)80049-F. [DOI] [Google Scholar]
- 6.Cavani F, Teles JH. Sustainability in catalytic oxidation: an alternative approach or a structural evolution? ChemSusChem. 2009;2:508–534. doi: 10.1002/cssc.200900020. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Aoki M, Noyori R. A “green” route to adipic acid: direct oxidation of cyclohexenes with 30 percent hydrogen peroxide. Science. 1998;281:1646–1647. doi: 10.1126/science.281.5383.1646. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, et al. Direct synthesis of adipic acid esters via palladium catalyzed carbonylation of 1,3-dienes. Science. 2019;366:1514–1517. doi: 10.1126/science.aaz1293. [DOI] [PubMed] [Google Scholar]
- 9.Beerthuis R, Rothenberg G, Shiju NR. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green. Chem. 2015;17:1341–1361. doi: 10.1039/C4GC02076F. [DOI] [Google Scholar]
- 10.Draths KM, Frost JW. Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 1994;116:399–400. doi: 10.1021/ja00080a057. [DOI] [Google Scholar]
- 11.Craft DL, Madduri KM, Eshoo M, Wilson CR. Identification and characterization of the CYP52 family of Candida tropicalis ATCC 20336, important for the conversion of fatty acids and alkanes to α, ω-dicarboxylic acids. Appl. Environ. Microbiol. 2003;69:5983–5991. doi: 10.1128/AEM.69.10.5983-5991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JL, Xia XX, Zhong JJ, Qian ZG. Direct biosynthesis of adipic acid from a synthetic pathway in recombinant Escherichia coli. Biotechnol. Bioeng. 2014;111:2580–2586. doi: 10.1002/bit.25293. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, et al. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway. Metab. Eng. 2018;47:254–262. doi: 10.1016/j.ymben.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Schrittwieser JH, Velikogne S, Hall M, Kroutil W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chem. Rev. 2018;118:270–348. doi: 10.1021/acs.chemrev.7b00033. [DOI] [PubMed] [Google Scholar]
- 15.France SP, Hepworth LJ, Turner NJ, Flitsch SL. Constructing biocatalytic cascades: in vitro and in vivo approach to de novo multi-enzyme pathways. ACS Catal. 2017;7:710–724. doi: 10.1021/acscatal.6b02979. [DOI] [Google Scholar]
- 16.Zhou H, et al. Chemo-and Regioselective dihydroxylation of benzene to hydroquinone enabled by engineered cytochrome P450 monooxygenase. Angew. Chem. Int. Ed. 2019;131:774–778. doi: 10.1002/ange.201812093. [DOI] [PubMed] [Google Scholar]
- 17.Song W, et al. Asymmetric assembly of high-value α-functionalized organic acids using a biocatalytic chiral-group-resetting process. Nat. Commun. 2018;9:3818. doi: 10.1038/s41467-018-06241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HL, et al. Bioamination of alkane with ammonium by an artificially designed multienzyme cascade. Metab. Eng. 2018;47:184–189. doi: 10.1016/j.ymben.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Li A, et al. Whole-cell-catalyzed multiple regio-and stereoselective functionalizations in cascade reactions enabled by directed. Evolution. Angew. Chem. Int. Ed. 2016;55:12026–12029. doi: 10.1002/anie.201605990. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, et al. Highly regio- and enantioselective multiple oxy- and aminofunctionalizations of alkenes by modular cascade biocatalysis. Nat. Commun. 2016;7:11917. doi: 10.1038/ncomms11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Both P, et al. Whole-cell biocatalysts for stereoselective C–H amination reactions. Angew. Chem. Int. Ed. 2016;55:1511–1513. doi: 10.1002/anie.201510028. [DOI] [PubMed] [Google Scholar]
- 22.Luo ZW, Lee SY. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nat. Commun. 2017;8:15689. doi: 10.1038/ncomms15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem. Soc. Rev. 2014;43:6954–6981. doi: 10.1039/C4CS00114A. [DOI] [PubMed] [Google Scholar]
- 24.Zhou K, Qiao K, Edgar S, Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015;33:377. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minty JJ, et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl Acad. Sci. USA. 2013;110:14592–14597. doi: 10.1073/pnas.1218447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minami H, et al. Microbial production of plant benzylisoquinoline alkaloids. Proc. Natl Acad. Sci. USA. 2008;105:7393–7398. doi: 10.1073/pnas.0802981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tassano E, Hall M. Enzymatic self-sufficient hydride transfer processes. Chem. Soc. Rev. 2019;48:5596. doi: 10.1039/C8CS00903A. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Li S, Liu L. Engineering redox balance through cofactor systems. Trends Biotechnol. 2014;6:337–343. doi: 10.1016/j.tibtech.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Turner NJ, O’Reilly E. Biocatalytic retrosynthesis. Nat. Chem. Biol. 2013;9:285–288. doi: 10.1038/nchembio.1235. [DOI] [PubMed] [Google Scholar]
- 30.Mutti FG, Knaus T, Scrutton NS, Breuer M, Turner NJ. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science. 2015;349:1525–1529. doi: 10.1126/science.aac9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Vlugt CJ, van der Werf MJ. Genetic and biochemical characterization of a novel monoterpene ɛ-lactone hydrolase from Rhodococcus erythropolis DCL14. Appl. Environ. Microbiol. 2001;67:733–741. doi: 10.1128/AEM.67.2.733-741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo EJ, et al. Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 2018;251:288–294. doi: 10.1016/j.biortech.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Q, Thomas SM, Kostichka K, Valentine JR, Nagarajan V. Genetic analysis of a gene cluster for cyclohexanol oxidation in Acinetobacter sp. Strain SE19 by in vitro transposition. J. Bacteriol. 2000;182:4744–4751. doi: 10.1128/JB.182.17.4744-4751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Wu S, Wu J, Li Z. Enantioselective cascade biocatalysis via epoxide hydrolysis and alcohol oxidation: one-pot synthesis of (R)-α-hydroxy ketones from meso- or racemic epoxides. ACS Catal. 2015;5:51–58. doi: 10.1021/cs5016113. [DOI] [Google Scholar]
- 35.Sattler JH, et al. Introducing an in situ capping strategy in systems biocatalysis to access 6-aminohexanoic acid. Angew. Chem. Int. Ed. 2014;53:14153–14157. doi: 10.1002/anie.201409227. [DOI] [PubMed] [Google Scholar]
- 36.Buntara T, et al. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. 2011;50:7083–7087. doi: 10.1002/anie.201102156. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez C, et al. Steric vs. electronic effects in the Lactobacillus brevis ADH-catalyzed bioreduction of ketones. Org. Biomol. Chem. 2014;12:673–681. doi: 10.1039/C3OB42057D. [DOI] [PubMed] [Google Scholar]
- 38.Opperman DJ, Reetz MT. Towards practical baeyer–villiger-monooxygenases: design of cyclohexanone monooxygenase mutants with enhanced oxidative stability. ChemBioChem. 2010;11:2589–2596. doi: 10.1002/cbic.201000464. [DOI] [PubMed] [Google Scholar]
- 39.Kohl A, Srinivasamurthy V, Bottcher D, Kabisch J, Bornscheuer UT. Co-expression of an alcohol dehydrogenase and a cyclohexanone monooxygenase for cascade reactions facilitates the regeneration of the NADPH cofactor. Enzym. Micro. Tech. 2018;108:53–58. doi: 10.1016/j.enzmictec.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Pennec A, Jacobs CL, Opperman DJ, Smit MS. Revisiting cytochrome P450-mediated oxyfunctionalization of linear and cyclic alkanes. Adv. Synth. Catal. 2015;357:118–130. doi: 10.1002/adsc.201400410. [DOI] [Google Scholar]
- 41.Karande R, et al. Continuous cyclohexane oxidation to cyclohexanol using a novel cytochrome P450 monooxygenase from Acidovorax sp. CHX100 in recombinant P. taiwanensis VLB120 biofilms. Biotechnol. Bioeng. 2016;113:52–61. doi: 10.1002/bit.25696. [DOI] [PubMed] [Google Scholar]
- 42.Karande R, Salamanca D, Schmid A, Buehler K. Biocatalytic conversion of cycloalkanes to lactones using an in-vivo cascade in Pseudomonas taiwanensis VLB120. Biotechnol. Bioeng. 2018;115:312–320. doi: 10.1002/bit.26469. [DOI] [PubMed] [Google Scholar]
- 43.Muschiol J, et al. An enzyme cascade synthesis of ε-caprolactone and its oligomers. Angew. Chem. Int. Ed. 2015;54:2784–2787. doi: 10.1002/anie.201410633. [DOI] [PubMed] [Google Scholar]
- 44.Omura T, Sato R. The carbon monoxide-biding pigment of liver microsomes. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 45.Krauser S, Kiefer P, Heinzle E. Multienzyme whole-cell in situ biocatalysis for the production of flaviolin in permeabilized cells of Escherichia coli. ChemCatChem. 2012;4:786–788. doi: 10.1002/cctc.201100351. [DOI] [Google Scholar]
- 46.Roell GW, et al. Engineering microbial consortia by division of labor. Microb. Cell Factories. 2019;18:35. doi: 10.1186/s12934-019-1083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X, Wang X, Chen T, Lu Y, Zhang H. Comparing E. coli mono-cultures and co-cultures for biosynthesis of protocatechuic acid and hydroquinone. Biochem. Eng. J. 2020;156:107518. doi: 10.1016/j.bej.2020.107518. [DOI] [Google Scholar]
- 48.Un Chae T, et al. Metabolic engineering for the production of dicarboxylic acids and diamines. Metab. Eng. 2020;58:2–16. doi: 10.1016/j.ymben.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Adkins J, Jordan J, Nielsen DR. Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5-aminovalerate and glutarate. Biotechnol. Bioeng. 2013;110:1726–1734. doi: 10.1002/bit.24828. [DOI] [PubMed] [Google Scholar]
- 50.Clomburg JM, et al. Integrated engineering of beta-oxidation reversal and omega-oxidation pathways for the synthesis of medium chain omega-functionalized carboxylic acids. Metab. Eng. 2015;28:202–212. doi: 10.1016/j.ymben.2015.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding author on reasonable request. Source data are provided with this paper.