Figure 1. Germline and Somatic Findings, by Gene.
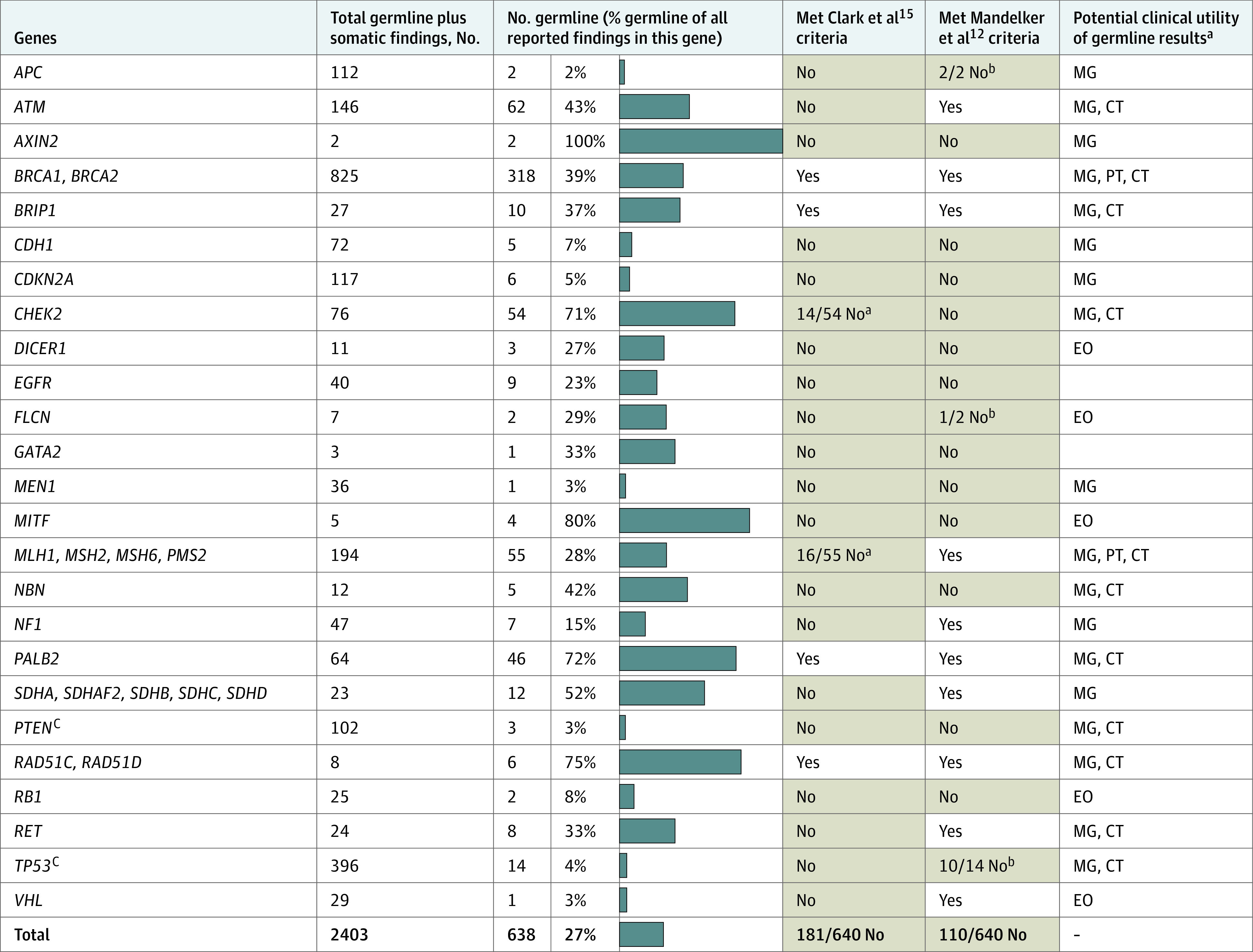
For each gene, the percentage of all reported findings that were of germline origin are shown by blue bars. Also indicated is whether the germline variants would (white background) or would not (light orange background) have met criteria for germline follow-up testing and whether the germline variants were potentially actionable. Only genes with at least 1 germline finding in this study are shown. Low-penetrance germline findings are excluded (eMethods in the Supplement). CT indicates gene-based clinical trial eligibility; EO, published expert opinion; MG, management guidelines; and PT, US Food and Drug Administration–approved precision-therapy labels. - indicates not applicable.
aThe Clark et al15 criteria include only certain variants in CHEK2 and exclude PMS2.
bThe Mandelker et al12 criteria for this gene specify patient age and/or tumor types.
cPTEN and TP53 frequently acquire somatic variations, which appear to be underreported in records available to this study (eResults in the Supplement).
