This randomized clinical trial assesses the efficacy and toxicity of radiotherapy plus icotinib compared with radiotherapy alone in older patients with unresectable esophageal squamous cell carcinoma (ESCC) in China.
Key Points
Question
Is icotinib plus radiotherapy superior to radiotherapy alone in patients with esophageal squamous cell carcinoma?
Findings
In this randomized clinical trial that included 127 patients 70 years or older with esophageal squamous cell carcinoma, patients treated with icotinib plus radiotherapy had a median overall survival of 24.0 months, whereas those treated with radiotherapy alone had a median overall survival of 16.3 months.
Meaning
In this study, icotinib plus radiotherapy was tolerable and improved overall survival in older patients with esophageal squamous cell carcinoma.
Abstract
Importance
Palliative radiotherapy (RT) is generally recommended for older patients with esophageal squamous cell carcinoma (ESCC) with poor prognosis. A new combination treatment is therefore needed.
Objective
To assess the efficacy and toxicity of RT plus icotinib vs RT alone in older patients with ESCC.
Design, Setting, and Participants
This randomized, multicenter, open-label, phase II clinical trial was conducted in China, with enrollment between January 1, 2015, and October 31, 2016. Patients aged 70 years or older with clinical stage T2 to T4, N0/1, M0/1a unresectable (because of comorbidities, T4 disease, unresectable lymph node, or refused surgery) ESCC were randomized 1:1 to receive RT plus icotinib or RT alone. Radiation was prescribed at 60 Gy in 30 fractions in both groups, and icotinib was administered at a dosage of 125 mg 3 times a day in the RT plus icotinib group. The last follow-up was completed on June 30, 2019, and data were analyzed from July 1 to September 30, 2019.
Interventions
Patients were randomized to either RT plus icotinib or RT alone.
Main Outcomes and Measures
The primary end point was overall survival (OS). Treatment-related toxic effects were evaluated. Immunohistochemistry was performed to analyze epidermal growth factor receptor (EGFR) expression if available.
Results
A total of 127 patients (median age, 76 years [range, 70-91 years]; 76 men [59.8%]) were enrolled and were eligible for survival analysis. Median OS was 24.0 (95% CI, 22.2-25.8) months in the RT plus icotinib group vs 16.3 (95% CI, 13.8-18.8) months in the RT group (hazard ratio, 0.53; 95% CI, 0.33-0.87; P = .008). No difference was observed in grades 3 or 4 adverse events. Patients with EGFR overexpression had a significantly better median overall survival (not reached vs 16.3 months [range, 2.6-45.1 months]; P = .03) in the RT plus icotinib group.
Conclusions and Relevance
In this randomized clinical trial, icotinib plus RT was well tolerated and improved OS in older patients with ESCC relative to RT alone. Patients with EGFR overexpression benefitted more from icotinib with RT.
Trial Registration
ClinicalTrials.gov Identifier: NCT02375581
Introduction
Concurrent chemoradiation therapy (CCRT) remains the standard therapy for unresectable esophageal squamous cell carcinoma (ESCC).1,2 However, its feasibility and efficiency for older patients are being challenged.3,4,5 Serious acute toxic effects, such as grade 3/4 hematologic toxicity, esophagitis, and pneumonitis, were commonly observed.4,6,7 In a retrospective analysis, only 33.3% of older patients completed chemoradiation, whereas 68.3% of nonelderly patients completed it.4 Treatment-related death was suspected in up to 18% of patients older than 75 years who underwent CCRT, even when both chemotherapy and radiotherapy (RT) were reduced in dose and field when necessary.8 Concurrent chemoradiation therapy showed no superiority over RT alone in survival.6 Palliative RT is generally recommended for older patients, but with limited survival benefit.1,9
Epidermal growth factor receptor (EGFR) is overexpressed in 30% to 70% of patients with ESCC and is associated with poor prognosis and an inferior response to conventional treatment.10 Several phase III studies have tried unsuccessfully to combine EGFR monoclonal antibody (cetuximab) with chemoradiation in esophageal cancer.11,12,13 An impaired overall survival (OS) was reported in the SCOPE1 trial,12 which may be partly due to the additional toxic effects. Thus, tolerance and toxicity should be carefully evaluated when delivering combined therapy, especially in older patients. The EGFR tyrosine kinase inhibitor (TKI) helps disrupt cell growth pathways and makes cells more sensitive to RT.14,15 Icotinib, an oral EGFR TKI, has been reported to markedly inhibit the proliferation of the human epidermoid squamous carcinoma A431 cell line with a high level of EGFR.16 Wang et al17 have evaluated the feasibility of icotinib in patients with advanced ESCC with EGFR overexpression. The efficacy and safety of combined EGFR inhibitors and RT has also been confirmed in patients with ESCC who are intolerant of CCRT.18,19 The pilot study of erlotinib plus RT revealed a 2-year survival rate of 44.4% with a reasonable safety profile.18
We therefore launched a randomized, multicenter, open-label, phase II clinical trial to investigate whether icotinib in combination with concurrent RT was superior to RT alone in older patients with ESCC.
Methods
Study Design
This randomized, multicenter, open-label, phase II clinical trial was conducted at Hangzhou Cancer Hospital, Huaian First People's Hospital and Lishui Central Hospital in China. The trial protocol (Supplement 1) was approved by the institutional review board and independent ethics committee of each participating center. All patients provided written informed consent. Patients’ records were anonymized and deidentified prior to analysis. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patient Eligibility
Eligible patients had histologically confirmed unresectable or medically inoperable ESCC clinically staged as T2 to 24, N0/1, M0/1a according to the 2002 International Union against Cancer TNM staging system and unsuitable for concurrent chemoradiation owing to comorbidities or patient choice.
Other eligibility criteria included age of 70 years or older; Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less; adequate renal, liver, and bone marrow reserve; and adequate nutritional intake.
Ineligibility criteria included history of other tumors, tracheoesophageal fistula, metastatic disease, severe dysphagia causing an inability to swallow icotinib, prior systemic treatment or thoracic radiation, and significant medical or psychiatric illnesses that would compromise the proposed treatment.
Randomization and Masking
Eligible patients were randomly assigned in a 1:1 ratio to receive RT plus icotinib or RT alone and stratified by T stage (T2 vs T3 vs T4), N stage (N0 vs N+), and ECOG performance status (0 or 1 vs 2). The minimization method was used to ensure balanced stratification.20 Central randomization was done with the use of the random number table method. Patients, study staff, and the sponsor were unmasked to treatment assignment.
Baseline Characteristics Evaluation
Age, sex, ECOG performance score, tumor stage, location, length, weight loss, and dysphagia were assessed. Degree of dysphagia was evaluated using the Atkinson scale (scores range from 0 to 4, with higher scores indicating greater dysphagia).21
Treatment Schedule
Radiotherapy was administered in both groups using high-energy linear accelerators with conventional fraction, 3-dimensional conformal technique or intensity-modulated RT. Gross tumor volume was defined as the primary tumor and any enlarged regional lymph nodes. Clinical target volume included the gross tumor volume plus a 5-cm expansion superiorly and inferiorly along the length of the esophagus and a 1-cm radial expansion and supraclavicular and mediastinal lymph node regions. Gross tumor volume was planned to receive 60 Gy (30 fractions at 2 Gy per fraction) and 40 Gy to clinical target volume (20 fractions at 2 Gy per fraction).
Icotinib was orally administrated as a 125-mg dose delivered 3 times per day concurrently with RT. Treatment was not interrupted unless stopped owing to excessive toxic effects, disease progression, or patient’s request.22
Dose Modifications
Acute and late radiation toxicity was scored according to the Radiation Therapy Oncology Group morbidity scoring criteria. Irradiation was interrupted for grade 3 or higher toxicity. Radiotherapy was restarted when toxicity recovered to grade 2 or lower. A 1-week treatment break from icotinib was required for grade 3 or higher toxicity. Interruptions were allowed for up to 2 weeks. Oral icotinib was restarted at full dose when toxicity resolved to grade 2 or lower.
EGFR Expression Assessment
Levels of EGFR expression were assessed using immunohistochemistry analysis in the central laboratory and scored independently by 2 pathologists who were blinded to clinical information using a 4-tier scoring scheme as follows: 0, no staining; 1+, faint cytoplasmic or/and membranous reactivity; 2+, moderate cytoplasmic and/or membranous reactivity; and 3+, strong cytoplasmic and/or membranous reactivity in at least 10% of tumor cells. If they could not reach agreement, a third pathologist helped with scoring. Tumors with a score of 3+ were interpreted as high expression (EGFR overexpression); others were interpreted as low EGFR expression.
Evaluation and Follow-up
All patients were hospitalized and assessed weekly during the treatment course or more often if clinically indicated. A history and physical examination, including a complete blood cell count were performed weekly, and serum chemistry was repeated every 2 weeks for toxicity assessment. The treatment toxicity was evaluated by the Common Toxicity Criteria version 4.0 and was recorded according to the worst score achieved during treatment.
Response of the primary tumor was determined 2 months after the completion of RT. Patients were scheduled to undergo neck-thorax-abdomen computed tomography and esophagogastroduodenoscopy. Follow-up was regularly carried out at 3-month intervals in the first 2 years, at 6-month intervals for 3 years, and then annually. Assessment was performed whenever disease progression was suspected. Esophageal recurrence was confirmed by histology or cytology. Treatment failure was defined as any sign of local or regional recurrence or distant metastasis.
Outcomes
The primary end point was OS, which was defined as the time from the date of group assignment to the date of death or the last follow-up (June 30, 2019). The secondary end points including progression-free survival (PFS) and treatment toxicity. Progression-free survival was measured from randomization until disease progression, death, or the date of the last follow-up.
Statistical Analysis
Data were analyzed from July 1 to September 30, 2019. Sample size was estimated according to the methodology by Lakatos.23,24 This trial was designed to have an 80% power to detect a 20% 2-year survival improvement (from 30% to 50%) with the addition of icotinib to concurrent RT,25 assuming an accrual time of 1 year, a minimum 2-year follow-up, and 5% missing data. The trial needed a recruitment of 127 patients (63 in the control group and 64 in the combination treatment group) with 80% power to detect an improvement in 2-year survival (Supplement 1).
All statistical analyses were performed using SPSS, version 20.0 (SPSS Inc). Efficacy and toxicity were evaluated in the intention-to-treat population. The χ2 test was used to evaluate differences of patient characteristics and toxicity. Survival analysis was performed using the Kaplan-Meier method. We did a stratified log-rank comparison of OS in the intention-to-treat population. Univariate and multivariate analyses with the Cox proportional hazards model were used to investigate the prognostic factors (TKI treatment, sex, age, T stage, N stage, ECOG performance status, tumor location, and tumor length) on survival. All statistical analyses were performed with a 2-sided P < .05 to indicate significance.
Results
Patient Characteristics
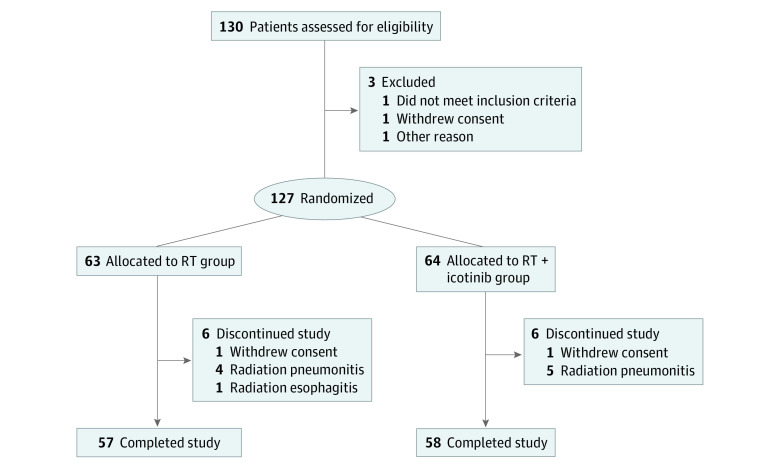
Between January 1, 2015, and October 31, 2016, 127 consecutive patients with newly diagnosed ESCC were enrolled in the trial; 63 were randomized to receive RT, and 64 were randomized to RT plus icotinib (Figure 1). Detailed demographic and baseline characteristics are listed in Table 1. The median age was 76 years (range, 70-91 years), 76 patients were men (59.8%), and 51 were women (40.2%). A total of 90 patients (70.9%) were considered malnourished (weight loss >10% of body weight). The clinical stage distribution was as follows: stage II, 65 (51.2%); stage III, 55 (43.3%); and stage IVA, 7 (5.5%). No demographic difference was observed between the 2 treatment groups.
Figure 1. CONSORT Diagram of Participant Flow Through the Trial.
Table 1. Baseline Patient Characteristics.
| Characteristic | No. of patients (%) | |
|---|---|---|
| RT (n = 63) | RT plus icotinib (n = 64) | |
| Age, y | ||
| ≤70-74 | 23 (36.5) | 29 (45.3) |
| ≥75-79 | 23 (36.5) | 22 (34.4) |
| ≥80 | 17 (27.0) | 13 (20.3) |
| Sex | ||
| Male | 36 (57.1) | 40 (62.5) |
| Female | 27 (42.9) | 24 (37.5) |
| ECOG performance status | ||
| 0-1 | 36 (57.1) | 31 (48.4) |
| 2 | 27 (42.9) | 33 (51.6) |
| T stage | ||
| T2 | 16 (25.4) | 17 (26.6) |
| T3 | 37 (58.7) | 35 (54.7) |
| T4 | 10 (15.9) | 12 (18.8) |
| N stage | ||
| N0 | 25 (39.7) | 27 (42.2) |
| N1 | 38 (60.3) | 37 (57.8) |
| M stage | ||
| M0 | 59 (93.7) | 61 (95.3) |
| M1a | 4 (6.3) | 3(4.7) |
| Clinical stage (UICC 2002) | ||
| IIA | 22 (34.9) | 21 (32.8) |
| IIB | 10 (15.9) | 12 (18.8) |
| III | 27(42.9) | 28 (43.8) |
| IVA | 4 (6.3) | 3 (4.7) |
| Tumor location | ||
| Cervical esophagus | 4 (6.3) | 6 (9.4) |
| Upper third | 17 (27.1) | 14 (21.9) |
| Middle third | 28 (44.4) | 31 (48.4) |
| Lower third | 14 (22.2) | 13 (20.3) |
| Tumor length, cm | ||
| <5 | 23 (36.5) | 31 (48.4) |
| ≥5 | 40 (63.5) | 33 (51.6) |
| Weight loss in 6 mo | ||
| ≤10% body weight | 22 (34.9) | 15 (23.4) |
| >10% body weight | 41 (65.1) | 49 (76.6) |
| Dysphagia, Atkinson scorea | ||
| 0-1 | 34 (54.0) | 37 (57.8) |
| ≥2 | 29 (46.0) | 27 (42.2) |
Abbreviations: ECOG, Eastern Cooperative Oncology; RT, radiation therapy.
Scores range from 0 to 4, with higher scores indicating greater dysphagia.
Treatment Compliance
The number of patients who completed per-protocol treatment was similar (57 [90.5%] in the RT group and 58 [90.6%] in the RT plus icotinib group). Nine patients in the RT group (14.3%) vs 10 in the RT plus icotinib group (15.6%) experienced a temporary interruption of RT with a median interruption of 4 days (range, 3-6 days). Four patients in the RT group (6.3%) compared with 5 in the RT plus icotinib group (7.8%) received less than 60 Gy of radiation dose owing to grade 3 radiation pneumonitis. Termination of RT at 40 Gy for grade 4 radiation esophagitis occurred in 1 patient (1.6%) in the RT group. One patient (1.6%) discontinued icotinib after 4 weeks owing to a swallowing problem and liver function damage.
Treatment-Related Toxicity
The incidence of treatment-related toxic effects is shown in Table 2. The majority of the adverse events were described as mild to moderate in severity. The most common toxic effects were radiation esophagitis (112 patients [88.2%]), neutropenia (69 patients [54.3%]), and radiation pneumonitis (63 patients [49.6%]) in both groups, without significant differences. Grade 3 or 4 toxic effects were observed in 17 (patients 26.6%) in the RT plus icotinib group vs 14 patients (22.2%) in the RT group (P = .50). No significant difference was found in radiation pneumonitis between the RT plus icotinib group (13 grade 1 cases [20.3%], 11 grade 2 [17.2%], 1 grade 3 [1.6%], and 0 grade 4) and the RT group (10 grade 1 cases [15.9%], 13 grade 2 [20.6%], and 0 grades 3 and 4) (P = .83); however, 1 patient in the RT group died from grade 5 radiation pneumonitis. Patients developed higher skin rash in the RT plus icotinib group (P = .01). Toxic effects were well controlled with appropriate dose reductions and supportive care.
Table 2. Treatment-Related Toxic Effects Compared Between the 2 Groups.
| Toxic effect | No. of patients (%) | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RT (n = 63)a | RT plus icotinib (n = 64) | ||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Hematologic toxicity | |||||||||
| Neutropenia | 14 (22.2) | 18 (28.6) | 2 (3.2) | 0 | 17 (26.6) | 16 (25) | 2 (3.1) | 0 | .94 |
| Thrombocytopenia | 10 (15.9) | 13 (20.6) | 0 | 0 | 13 (20.3) | 11 (17.2) | 1 (1.6) | 0 | .48 |
| Anemia | 9 (14.3) | 3 (4.8) | 0 | 0 | 8 (12.5) | 3 (4.7) | 0 | 0 | .96 |
| Nonhematologic toxicity | |||||||||
| Radiation esophagitis | 24 (38.1) | 23 (36.5) | 7 (11.1) | 1 (1.6) | 28 (43.8) | 21 (32.8) | 8 (12.5) | 0 | .82 |
| Radiation pneumonitis | 20 (31.7) | 7 (11.1) | 3 (4.8) | 0 | 19 (29.7) | 9 (14.1) | 4 (6.3) | 1 (1.6) | .83 |
| Nausea/vomiting | 20 (31.7) | 5 (7.9) | 0 | 0 | 24 (37.5) | 4 (6.3) | 0 | 0 | .77 |
| ALT/AST elevation | 7 (11.1) | 4 (6.3) | 0 | 0 | 9 (14.1) | 4 (6.3) | 1 (1.6) | 0 | .73 |
| Skin rash | 5 (7.9) | 1 (1.6) | 0 | 0 | 15 (23.4) | 5 (7.8) | 0 | 0 | .01 |
| Diarrhea | 6 (9.5) | 2 (3.2) | 0 | 0 | 9 (14.1) | 4 (6.3) | 0 | 0 | .45 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; RT, radiation therapy.
One patient (1.6%) experienced grade 5 radiation pneumonitis after a radiation of 54 Gy in the RT group.
Survival Analysis
The median follow-up time was 42.8 months (interquartile range, 42.1-43.6 months) for all patients. The overall response rate was 84.4% in the RT plus icotinib group and 60.3% in the RT group. Patients in the RT plus icotinib group had a significantly better survival compared with their RT counterparts (hazard ratio, 0.53; 95% CI, 0.33-0.87; P = .008. Figure 2A), with a median OS of 24.0 (95% CI, 22.2-25.8) months vs 16.3 (95% CI, 13.8-18.8) months and 2-year OS of 43.7% (95% CI, 29.2%-65.4%) vs 29.6% (95% CI, 19.4%-49.1%). Median PFS was 22.7 (95% CI, 18.3-27.1) months in the RT plus icotinib group and 14.3 (95% CI, 10.7-17.9) months in the RT group (Figure 2B). Patients in the RT plus icotinib group had a significantly longer PFS (hazard ratio, 0.58; 95% CI, 0.37-0.92; P = .02).
Figure 2. Kaplan-Meier Analyses of Survival in the Intention-to-Treat Population.
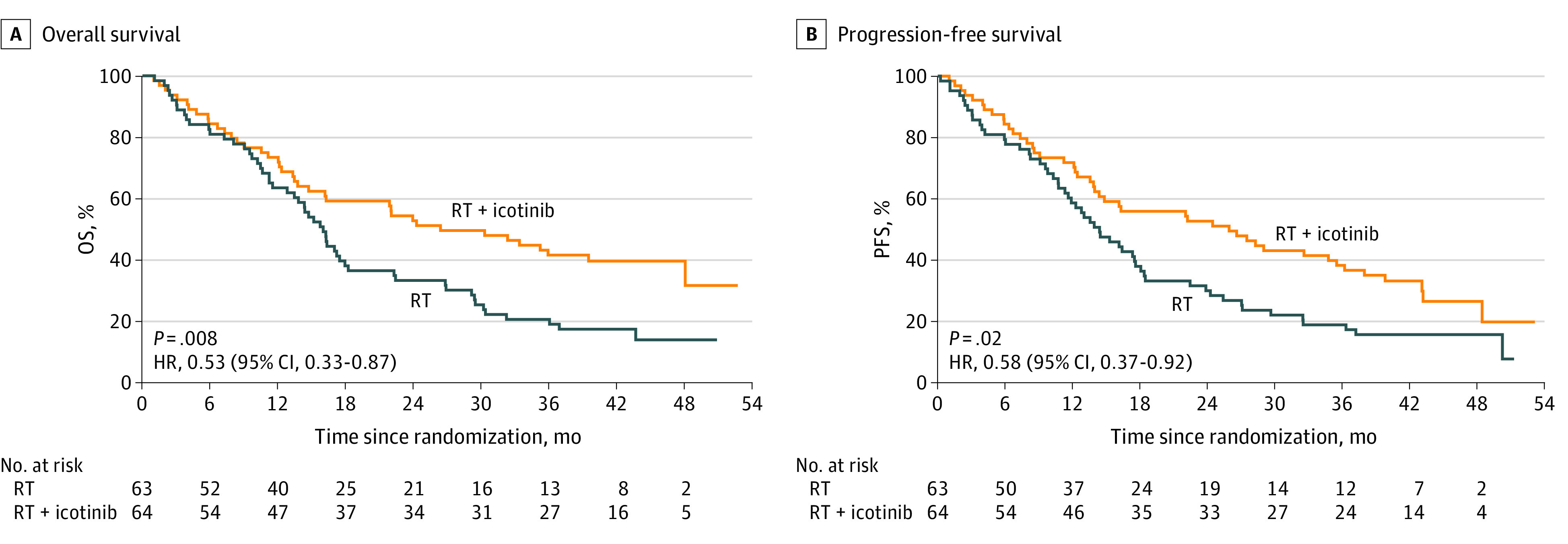
HR indicates hazard ratio; OS, overall survival; PFS, progression-free survival; and RT, radiotherapy.
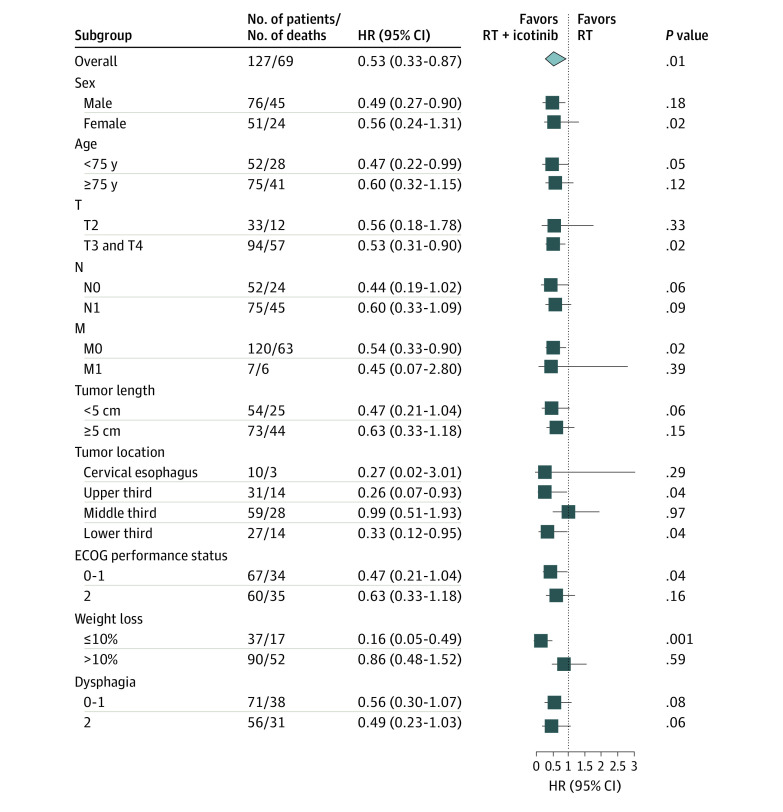
The survival benefit of icotinib added to RT was evident in all subgroups examined (Figure 3). By univariate and multivariate analysis, use of icotinib was an independent prognostic factor (eTables 1 and 2 in Supplement 2).
Figure 3. Subgroup Analysis of Icotinib Benefit for Overall Survival.
HR indicates hazard ratio; RT, radiotherapy.
Expression of EGFR Protein
In the post hoc analysis, EGFR protein expression was evaluated in 61 patients (48.0%) from whom there were sufficient pretreatment tumor tissues for immunohistochemistry, including 32 (50%) from the RT plus icotinib group and 29 (46%) from the RT group. In the final analysis, no expression of EGFR, 1+ EGFR expression, 2+ EGFR expression, and 3+ EGFR expression were observed in 5, 6, 14, and 7 patients in the RT plus icotinib group and in 11, 2, 15, and 1 patient in RT group, respectively. In the RT plus icotinib group, median OS was not reached in the EGFR overexpression group and was 16.3 months (range, 2.6-45.1 months) in the low EGFR expression group (P = .03) (eFigure 1 in Supplement 2). In the RT group, median OS was 16.2 months (range, 2.6-45.1 months) in patients with low EGFR expression, and the 1 patient with EGFR overexpression lived 32.3 months with no disease progression (eFigure 2 in Supplement 2).
Patterns of Failure
The crude patterns of primary failure are shown in eTable 3 in Supplement 2. Twenty-six patients (40.6%) in the RT plus icotinib group experienced disease recurrence compared with 41 (65.1%) in the RT group. The incidence of local or regional recurrence was significantly lower in the RT plus icotinib group (26 patients [40.6%]) than the RT group (38 patients [60.3%]) (P = .03). Adding icotinib to RT diminished the rate of distant metastasis (6.3% vs 15.9%, respectively; P = .08), but this finding was not statistically significant.
Discussion
Because of their reduced physiologic reserve of body function and highly prevalent comorbidities, older patients have poor tolerance for CCRT and are at increased risk of toxic effects. Therefore, palliative RT has been generally recommended. Modern approaches to cancer treatment focus on combination strategies involving targeted therapy and RT. The essential hypothesis of the present trial was that icotinib concurrently administered with RT would promote local and regional effects as well as diminish distant metastases.
Our trial found that patients who received concurrent icotinib with RT had significantly longer OS and PFS than those who received RT alone. The OS benefit persisted across all subgroups. The available data suggest that the combination resulted in not only less local and regional recurrence but also fewer distant metastases at 2 years.
Few studies have reported the application of RT plus EGFR TKIs in older patients with esophageal cancer.26,27 The combination of gefitinib plus RT for elderly patients was reported by Xu and colleagues.26 Their results showed a median OS of 14.0 months, with 5 of 20 patients experiencing severe toxic effects.26 In a pilot study18 evaluating the efficacy of concurrent erlotinib and RT for chemoradiotherapy-intolerant patients with ESCC, the median OS was 21.1 months, which is similar to our experimental group. Inconsistent with our results, RTOG 0436 trial13 failed to demonstrate the superiority of the addition EGFR inhibitor to CCRT. We consider that this negative result may be partly due to the higher incidence of toxicity caused by the intensified treatment with CCRT and cetuximab. In addition, unlike our study, RTOG 0436 enrolled patients with a mixture of squamous cell cancer and adenocarcinoma, which may weaken the benefits of cetuximab.13
Median survival and 2-year survival in the RT group in our trial was higher compared with the RT-alone arm of RTOG 85-01 (16.3 vs 8.9 months, 29.6% vs 10%).28 This finding may be partly owing to the progress of modern technique and greater experience in radiation management. Our results that favored adding icotinib to RT did not depend on worsened data in the control group.
The ARTDECO study29 explored definitive radiotherapy concurrent with carboplatin and paclitaxel in locally advanced esophageal cancer and demonstrated a 3-year local progression-free interval of 77% for ESCC and a 3-year OS rate of 40%. The median age of these patients was 70 years. These findings suggested the safety and tolerability of CCRT in older patients. However, in our study, enrolled patients were older than in the ArtDeco trial. In the present study, more than 70% of patients were malnourished. These differences may contribute to the difference in CCRT tolerance.
Our study showed that this combined therapy was generally well tolerated in patients with ESCC. The toxic effects associated with icotinib and RT did not overlap. The addition of icotinib did not increase other toxic reactions except for mild skin rash and diarrhea. The safety profile of icotinib observed in this trial was consistent with that seen previously with EGFR TKIs for the treatment of esophageal cancer.17,30 The most common radiation toxic effects were esophagitis and pneumonitis, which were similar to historical data.30 Few cases of grade 3 or 4 hematologic toxic effects were observed in our trial. However, grade 3 or 4 hematologic toxic effects have been commonly reported in patients treated with CCRT.4,6 No significant difference was found in radiation pneumonitis between the RT plus icotinib group and RT group. This finding is in accordance with previous reports.31,32,33 Therefore, the combination of icotinib and RT had a more favorable toxicity profile than did CRT.
A previous study has evaluated the feasibility of icotinib in patients with advanced ESCC with EGFR overexpression.17 The response rate was higher (17.6% vs 0%, P = .34) for patients with high EGFR-expressing tumors. Furthermore, all patients who responded to icotinib showed EGFR overexpression. Another study suggested that EGFR amplification appears to identify a subgroup of patients with esophageal cancer who may benefit from gefitinib.34 Few studies evaluated the association between EGFR expression and survival parameters in EGFR TKIs combined with RT.35 Our study found that in the RT plus icotinib group, patients with EGFR overexpression had a significantly better OS (not reached vs 16.3 months, P = .03). In addition, EGFR expression could help identify patients who will benefit more from the combination of EGFR TKIs and RT.
Limitations
Our study has some limitations. Tumor tissues were obtained from only 61 patients for EGFR expression assessment. Analysis of EGFR expression was conducted with a limited sample size, and we did not include EGFR status as part of the eligibility criteria. We analyzed the data at a median follow-up of approximately 2 years. Long-term follow-ups are warranted to evaluate the toxic effects and efficacy. Icotinib is not currently available outside China.
Conclusions
The findings of this study suggest that concurrent therapy with icotinib and RT was well tolerated and tended to improve survival in older patients with ESCC. Patients with EGFR overexpression seemed to benefit more from icotinib with RT. A phase III trial to further identify the efficacy of icotinib with RT is forthcoming.
Trial Protocol
eFigure 1. Overall Survival in RT + Icotinib Group
eFigure 2. Overall Survival in RT Group
eTable 1. Univariate Analysis of Potential Factors Associated With Survival in all ESCC Patients
eTable 2. Multivariate Analysis of Prognostic Factors in all ESCC Patients
eTable 3. Patterns of First Failure Between Two Groups of Elderly ESCC Patients
Data Sharing Statement
References
- 1.Herskovic A, Martz K, al-Sarraf M, et al. . Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus.. N Engl J Med. 1992;326(24):1593-1598. doi: 10.1056/NEJM199206113262403 [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Pajak TF, Ginsberg RJ, et al. . INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167-1174. doi: 10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 3.Tougeron D, Di Fiore F, Thureau S, et al. . Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer. 2008;99(10):1586-1592. doi: 10.1038/sj.bjc.6604749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi S, Ohtsu A, Doi T, et al. . A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol. 2007;30(6):607-611. doi: 10.1097/COC.0b013e3180ca7c84 [DOI] [PubMed] [Google Scholar]
- 5.Tao S, Zhang X, Min F, Wu S. Concurrent chemoradiotherapy using paclitaxel plus cisplatin in the treatment of elderly patients with esophageal cancer. Onco Targets Ther. 2015,8:3087-3094. doi: 10.2147/OTT.S92537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Xi M, Zhao L, et al. . Is there a benefit in receiving concurrent chemoradiotherapy for elderly patients with inoperable thoracic esophageal squamous cell carcinoma? PLoS One. 2014;9(8):e105270. doi: 10.1371/journal.pone.0105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Z, Tian Y, He B, et al. . Associated factors of radiation pneumonitis induced by precise radiotherapy in 186 elderly patients with esophageal cancer. Int J Clin Exp Med. 2015;8(9):16646-16651. [PMC free article] [PubMed] [Google Scholar]
- 8.Wakui R, Yamashita H, Okuma K, et al. . Esophageal cancer: definitive chemoradiotherapy for elderly patients. Dis Esophagus. 2010;23(7):572-579. doi: 10.1111/j.1442-2050.2010.01062.x [DOI] [PubMed] [Google Scholar]
- 9.Ajani JA, D’Amico TA, Almhanna K, et al. ; National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(2):194-227. doi: 10.6004/jnccn.2015.0028 [DOI] [PubMed] [Google Scholar]
- 10.Pande AU, Iyer RV, Rani A, et al. . Epidermal growth factor receptor-directed therapy in esophageal cancer. Oncology. 2007;73(5-6):281-289. doi: 10.1159/000132393 [DOI] [PubMed] [Google Scholar]
- 11.Ruhstaller T, Thuss-Patience P, Hayoz S, et al. ; Swiss Group for Clinical Cancer Research (SAKK); German Esophageal Cancer Study Group; Austrian ‘Arbeitsgemeinschaft Medikamentöse Tumortherapie’ (AGMT); Fédération Francophone de Cancérologie Digestive (FFCD)/Fédération de Recherche en Chirurgie (FRENCH) . Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann Oncol. 2018;29(6):1386-1393. doi: 10.1093/annonc/mdy105 [DOI] [PubMed] [Google Scholar]
- 12.Crosby T, Hurt CN, Falk S, et al. . Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14(7):627-637. doi: 10.1016/S1470-2045(13)70136-0 [DOI] [PubMed] [Google Scholar]
- 13.Suntharalingam M, Winter K, Ilson D, et al. . Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the nrg oncology rtog 0436 phase 3 randomized clinical trial. JAMA Oncol. 2017;3(11):1520-1528. doi: 10.1001/jamaoncol.2017.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinnaiyan P, Huang S, Vallabhaneni G, et al. . Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328-3335. doi: 10.1158/0008-5472.CAN-04-3547 [DOI] [PubMed] [Google Scholar]
- 15.Tortora G, Gelardi T, Ciardiello F, Bianco R. The rationale for the combination of selective EGFR inhibitors with cytotoxic drugs and radiotherapy. Int J Biol Markers. 2007;22(1 suppl 4):S47-S52. doi: 10.1177/17246008070221s406 [DOI] [PubMed] [Google Scholar]
- 16.Tan F, Shen X, Wang D, et al. . Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012;76(2):177-182. doi: 10.1016/j.lungcan.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Niu H, Fan Q, et al. . Predictive value of EGFR overexpression and gene amplification on icotinib efficacy in patients with advanced esophageal squamous cell carcinoma. Oncotarget. 2016;7(17):24744-24751. doi: 10.18632/oncotarget.8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai Y, Hui Z, Wang J, et al. . Concurrent erlotinib and radiotherapy for chemoradiotherapy-intolerant esophageal squamous cell carcinoma patients: results of a pilot study. Dis Esophagus. 2013;26(5):503-509. doi: 10.1111/j.1442-2050.2012.01380.x [DOI] [PubMed] [Google Scholar]
- 19.Liang J, E M, Wu G, et al. . Nimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a phase ii clinical trial. Onco Targets Ther. 2013;6:1589-1596. doi: 10.2147/OTT.S50945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 21.Atkinson M. Diseases of the alimentary system; dysphagia. Br Med J. 1977;1(6053):91-93. doi: 10.1136/bmj.1.6053.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao-Lu YU, Wang HM, Wang WM. Efficacy of icotinib as first-line therapy for 134 patients with advanced lung adenocarcinoma of positive EGFR mutation. J Chinese Oncol. 2017;23(9):772-775. doi: 10.11735/j.issn.1671-170X.2017.09.B006 [DOI] [Google Scholar]
- 23.Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics. 1988;44(1):229-241. doi: 10.2307/2531910 [DOI] [PubMed] [Google Scholar]
- 24.Lakatos E. Designing complex group sequential survival trials. Stat Med. 2002;21(14):1969-1989. doi: 10.1002/sim.1193 [DOI] [PubMed] [Google Scholar]
- 25.D'Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis. 2014;6(suppl 2):S253-S264. doi: 10.3978/j.issn.2072-1439.2014.04.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Zheng Y, Sun X, et al. . Concurrent radiotherapy with gefitinib in elderly patients with esophageal squamous cell carcinoma: preliminary results of a phase II study. Oncotarget. 2015;6(35):38429-38439. doi: 10.18632/oncotarget.5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer R, Chhatrala R, Shefter T, et al. . Erlotinib and radiation therapy for elderly patients with esophageal cancer–clinical and correlative results from a prospective multicenter phase 2 trial. Oncology. 2013;85(1):53-58. doi: 10.1159/000351617 [DOI] [PubMed] [Google Scholar]
- 28.Cooper JS, Guo MD, Herskovic A, et al. ; Radiation Therapy Oncology Group . Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999;281(17):1623-1627. doi: 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 29.Hulshof MCCM, Geijsen D, Rozema T, et al. . A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J Clin Oncol, 2020,38(suppl 4):281. doi: 10.1200/JCO.2020.38.4_suppl.281 [DOI] [PubMed] [Google Scholar]
- 30.Zhang XB, Xie CY, Li WF, Zhang P, Wu SX. [Phase II study of radiotherapy plus erlotinib for elder patients with esophageal carcinoma]. Zhonghua Yi Xue Za Zhi. 2012;92(23):1615-1617. [PubMed] [Google Scholar]
- 31.Niho S, Ohe Y, Ishikura S, et al. . Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol. 2012;23(9):2253-2258. doi: 10.1093/annonc/mds012 [DOI] [PubMed] [Google Scholar]
- 32.Komaki R, Allen PK, Wei X, et al. . Adding erlotinib to chemoradiation improves overall survival but not progression-free survival in stage iii non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;92(2):317-324. doi: 10.1016/j.ijrobp.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ready N, Jänne PA, Bogart J, et al. ; Cancer, Leukemia Group B, Chicago, IL . Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol. 2010;5(9):1382-1390. doi: 10.1097/JTO.0b013e3181eba657 [DOI] [PubMed] [Google Scholar]
- 34.Petty RD, Dahle-Smith A, Stevenson DAJ, et al. . Gefitinib and egfr gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279-2287. doi: 10.1200/JCO.2016.70.3934 [DOI] [PubMed] [Google Scholar]
- 35.Mehta VK. Radiotherapy and erlotinib combined: review of the preclinical and clinical evidence. Front Oncol. 2012;2:31. doi: 10.3389/fonc.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Overall Survival in RT + Icotinib Group
eFigure 2. Overall Survival in RT Group
eTable 1. Univariate Analysis of Potential Factors Associated With Survival in all ESCC Patients
eTable 2. Multivariate Analysis of Prognostic Factors in all ESCC Patients
eTable 3. Patterns of First Failure Between Two Groups of Elderly ESCC Patients
Data Sharing Statement