Abstract
Rituximab targets the CD20 antigen expressed on B-lymphocytes and is used to treat recurrent minimal change disease, but experience of its use in pregnancy is limited. We describe a 28-year-old Caucasian female, with recurrent nephrotic syndrome secondary to minimal change disease. She had failed to respond to non-teratogenic alternative therapies. The patient was successfully maintained in remission with rituximab during two consecutive pregnancies. Rituximab (1 g) was administered at 14+6 weeks 14 weeks and 6 days during Pregnancy 1 and 500 mg administered at 23+4 weeks 23 weeks and 4 days of Pregnancy 2. Rituximab had no apparent effect on infant B-cell development in either pregnancy, as neonatal lymphocyte titres were within normal range. There were no maternal complications in either pregnancy. Neither infant encountered infection-related complications. Although rituximab administration during pregnancy appeared safe, evidence of placental transfer is reported with neonatal B-cell depletion, thus alternatives with known safety profiles in pregnancy should be considered before rituximab administration.
Keywords: Rituximab, pregnancy, minimal change nephropathy
Introduction
Rituximab is a chimeric monoclonal antibody, which targets the CD20 antigen expressed on B lymphocytes,1 and is used to treat numerous immunologically mediated conditions. Rituximab is not currently licensed as a therapy for minimal change disease (MCD) due to limited evidence of its safety in randomised controlled trials and a lack of guidelines published by national or international societies. However, rituximab has been proposed to reduce frequency of relapses, sustaining steroid-free remission in steroid-dependent patients with recurrent MCD.2–5 Dosing regimens vary, including two 1 g doses given 3 to 6 months apart, to 375 mg/m2 body surface area once weekly for four weeks.2,5,6 Experience of rituximab use in pregnancy is limited, particularly for the treatment of MCD. Here, we report a patient with recurrent relapsing MCD, who was maintained in remission with rituximab during two consecutive pregnancies.
Case
A 28-year-old Caucasian female presented with recurrent relapsing nephrotic syndrome at age 3, confirmed on renal biopsy to be MCD at age 11, and had been treated with long-term immunosuppression therapies including cyclophosphamide, calcineurin inhibitors (ineffective), mycophenolate mofetil (MMF) and frequent courses of high-dose prednisolone resulting in steroid-induced osteopenia and short stature (height: 1.47 m). Her disease had been maintained in remission with MMF, which was stopped after pregnancy was confirmed in November 2012. However, her disease rapidly relapsed and she subsequently miscarried. She was reluctant to restart MMF and was offered rituximab (1 g) in February 2016, and her disease went into complete remission with a planned second dose in 3 to 6 months.
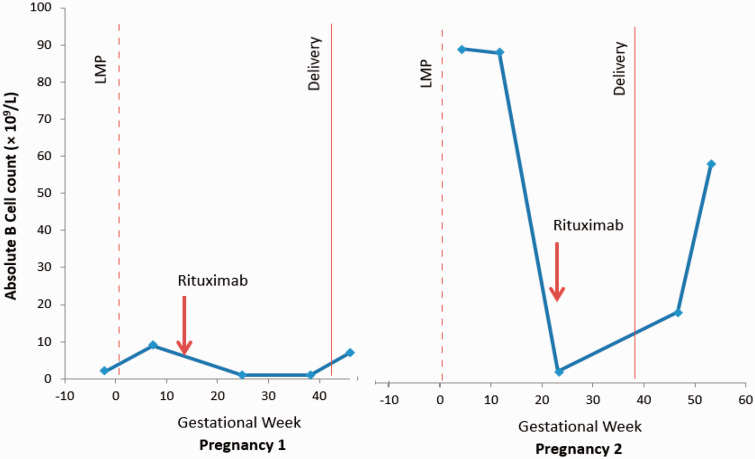
Pregnancy 1
She reported an unplanned pregnancy in May 2016. Following her reluctance to consider a further course of high-dose steroids and concerns of a high-risk of nephrotic relapse during pregnancy, a decision was made to monitor B-lymphocytes and give a dose if there were signs of restoration. Absolute B-cell counts are displayed in Figure 1. At 14 weeks and 6 days of gestation, rituximab 1 g was administered. The patient was counselled about possible fetal B-lymphocyte depletion, the need for neonatal monitoring and potential future unknown risks of immunological disease.
Figure 1.
Absolute B-cell count during pregnancies 1 and 2 according to gestational week. LMP: last menstrual period.
Blood pressure, proteinuria and renal function were normal throughout pregnancy as was fetal monitoring. Blood pressure measurements ranged from systolic 84 to 104 mmHg and diastolic 50 to 67 mmHg, creatinine ranged from 35 to 47 μmol/L, urea ranged from 2.2 to 3.3 mmol/L and urine protein/creatinine ratio remained within normal range. After spontaneous onset of labour at 41 weeks, she underwent an emergency caesarean section for failure to progress. There were no perinatal or postpartum complications, and future contraception was recommended.
The healthy neonate weighed 3800 g (75th centile). Neonatal lymphocyte subsets taken at Day 1 were normal (Table 1). She decided not to breastfeed for personal reasons, although the minimal risk of neonatal absorption was discussed. A normal schedule of neonatal vaccination was recommended on paediatric immunology advice; however, the patient declined vaccinations due to concerns regarding side effects.
Table 1.
Lymphocyte subset levels at birth for each neonate.
| CD4 cells (cells/µL) NR 400–3500 | CD3 (%) NR 28–76 | CD3 cells (cells/µL) NR 600–5000 | CD4 (%) NR 17–52 | B cells (%) NR 5–22 | B-cell absolute counts (cells/µL)NR 40–1100 | NK (%) NR 6–58 | NK cells (cells/µL) NR 100–1900 | LSUM | CD8 cells (cells/µL) NR 200–1900 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy 1 | 2303 | 52 | 3551 | 36% | 15 | 1087 | 23 | 1672 | 6310 | 1093 |
| Pregnancy 2 | 1499 | 88 | 2183 | 58% | 6 | 144 | 1 | 39 | 2366 | 738 |
NR: normal range; NK: natural killer; LSUM: lymphocyte summation.
Pregnancy 2
A second unplanned pregnancy was confirmed in July 2017, approximately 18 weeks after her previous delivery. Remission had been maintained and an expectant approach with B-cell monitoring was planned again. Rituximab (500 mg) was administered at 23 weeks and 4 days of gestation, (Figure 1). She was admitted for intravenous fluids following an episode of dehydration at 28+1 weeks’ gestation, with no underlying cause identified. All laboratory parameters and fetal ultrasound were normal and remained reassuring. Blood pressure ranged from systolic 80 to 114 mmHg and diastolic 50 to 61 mmHg, creatinine from 38 to 52 μmol/L, urea from 1.9 to 3.9 mmol/L and urinary protein/creatinine ratio from 3.5 to 9.0 mg/mmol. Trial of vaginal delivery was offered, and the patient chose elective caesarean section which was performed at 39 weeks (birth weight 3245 g, 40th centile).
Neonatal lymphocyte subsets were in normal range (Table 1) at Day 3, although natural killer (NK) cells were reduced. Repeat testing prior to vaccination was recommended but was declined. The mother remains in remission.
Discussion
Rituximab use in pregnancy is not currently approved by the United States Food and Drug Administration due to pre-clinical animal studies demonstrating adverse effects on the fetus7 and insufficient human controlled studies. Thus, rituximab should only be used in pregnancy if the benefit outweighs the risk, and women of childbearing potential are recommended to use effective contraception throughout therapy and for 12-months post-treatment.8,9 In this case, due to high-risk of relapse of MCD, maternal reluctance for use of high-dose steroids and a lack of alternative effective non-teratogenic therapies, rituximab was used successfully in two pregnancies to maintain remission. The first pregnancy dose (1 g) was given in keeping with previous studies (two doses of 1 g 6 months apart), and when B cells were repopulating.2 Given the high B-cell recovery early in the second pregnancy, which is associated with relapse, a further maintenance dose was administered. There was a delay with dosing in the second pregnancy for logistical reasons, and a reduced dose (500 mg) was given due to concerns about placental transfer leading to higher neonatal concentrations due to exposure in later pregnancy.
Rituximab had no apparent effect on infant B-cell development in either pregnancy, as neonatal lymphocyte titres were within normal range and no infection-related complications occurred, although neonatal NK cells were low on one occasion.
A recent study identified 92 pregnancies with maternal rituximab exposure using a global drug safety database which did not end in iatrogenic or spontaneous pregnancy loss. There was one maternal death, one stillbirth, two congenital abnormalities (cardiac malformation and talipes equinovarus), four neonatal infections and eleven neonates had haematological abnormalities (peripheral B-cell depletion, neutropenia, lymphopenia, thrombocytopenia and anaemia) but without infective complications.10
Other case reports have described a higher titre of immature subsets of B lymphocytes (CD19+CD79a+), but with no clinical consequences after rituximab use in pregnancy,11 reduced neonatal B-lymphocyte count at delivery with resolution by six-months with third trimester use12 and normal neonatal outcomes.13,14 However, there may be undetectable changes to other components of the immune system, and vigilance for infection is necessary.
There is a paucity of data on pregnancies in women with nephrotic syndrome; however, relapse appears to be associated with adverse outcomes and should be avoided.15 An alternative strategy in this case would have been reactive treatment with high-dose corticosteroids, which the mother was extremely reluctant to consider. An increased risk of congenital abnormality has been refuted,16 and no association between high-dose corticosteroids and preterm deliveries and low birthweight has been demonstrated in women with Crohn’s disease, rheumatoid arthritis and systemic lupus erythematosus.17–19 High corticosteroid doses in pregnancy, however, have been associated with an increase in number and severity of neonatal infections,20 gestational diabetes, mellitus established maternal non-pregnancy-related side effects and may also be associated with an increased risk of cardiovascular and metabolic disease in adulthood.21
In summary, we report two successful pregnancies in a woman with steroid-dependent MCD treated with rituximab. Until further safety data are known, rituximab administration during pregnancy to maintain remission for women with MCD should be reserved for those with no alternative treatment strategies.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Written consent for publication has been obtained from the patient.
Guarantor
KB
Contributorship
FH, KB and KB meet the full authorship criteria and wrote the manuscript together.
References
- 1.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000; 95: 3900–3908. [PubMed] [Google Scholar]
- 2.Papakrivopoulou E, Shendi A, Salama A, et al. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology (Carlton) 2016; 21: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014; 25: 850–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takura T, Takei T, Nitta K. Cost-effectiveness of administering rituximab for steroid-dependent nephrotic syndrome and frequently relapsing nephrotic syndrome: a preliminary study in Japan. Sci Rep 2017; 7: 46036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronbichler A, Kerschbaum J, Fernandez-Fresnedo G, et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 2014; 39: 322–330. [DOI] [PubMed] [Google Scholar]
- 6.Brown L, Jobson M, Payan Schober F, et al. The evolving role of rituximab in adult minimal change glomerulopathy. Am J Nephrol 2017; 45: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidyanathan A, McKeever K, Anand B, et al. Developmental immunotoxicology assessment of rituximab in cynomolgus monkeys. Toxicol Sci 2011; 119: 116–125. [DOI] [PubMed] [Google Scholar]
- 8.United States Food and Drug Administration. Rituxan final labeling text, www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf (2010, accessed 7 November 2018).
- 9.US National Library of Medicine. Drugs and lactation: database (lactmed). TOXNET, https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm (2017, accessed 7 November 2018).
- 10.Chakravarty E, Murray E, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011; 117: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Martínez M, Baranda-Cándido L, González-Amaro R, et al. Modified neonatal B-cell repertoire as a consequence of rituximab administration to a pregnant woman. Rheumatology (Oxford ) 2013; 52: 405–406. [DOI] [PubMed] [Google Scholar]
- 12.Klink D, van Elburg R, Schreurs M, et al. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol 2008; 271363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojeda-Uribe M, Gilliot C, Jung G, et al. Administration of rituximab during the first trimester of pregnancy without consequences for the newborn. J Perinatol 2006; 26: 252–255. [DOI] [PubMed] [Google Scholar]
- 14.Kimby E, Sverrisdottir A, Elinder G. Safety of rituximab therapy during the first trimester of pregnancy: a case history. Eur J Haematol 2004; 72: 292–295. [DOI] [PubMed] [Google Scholar]
- 15.Hall M, Brunskill N. Glomerulonephritis and the nephrotic syndrome in pregnancy. Fet Matern Med Rev 2010; 21: 163–184. [Google Scholar]
- 16.Skuladottir H, Wilcox A, Ma C, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res Part A Clin Mol Teratol 2014; 100: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Arfaj A, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus 2010; 19: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 18.de Man Y, Hazes J, van der Heide H, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum 2009; 60: 3196–3206. [DOI] [PubMed] [Google Scholar]
- 19.Nørgård B, Pedersen L, Christensen L, et al. Therapeutic drug use in women with Crohn's disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol 2007; 102: 1406–1413. [DOI] [PubMed] [Google Scholar]
- 20.Desai R, Bateman B, Huybrechts K, et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ 2017; 356: j895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seckl J, Holmes M. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab 2007; 3: 479–488. [DOI] [PubMed] [Google Scholar]