Acute kidney injury (AKI) is a frequent secondary feature in coronavirus disease 2019 (COVID-19) patients [1]. AKI may require renal replacement therapy, complicates management and is associated with impaired outcomes. The absence of current drug treatments for COVID-19 and associated AKI renders patients dependent on supportive intensive care, and therefore new therapeutic approaches are urgently needed [1].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry into human host cells requires endocytosis mechanisms activated by binding of SARS-CoV-2 spike protein (S) to angiotensin-converting enzyme 2 (ACE2) on the cell surface [2]. Thus, blockade of this binding step is a potential therapeutic approach.
ACE2 is located in cholesterol rafts. Cilastatin is a specific competitive blocker of renal dehydropeptidase I (DHP-I) enzyme, which is anchored to cholesterol rafts of renal tubular cells. Cilastatin protects both in vitro and in vivo against tubular cell injury and AKI induced by nephrotoxic drugs such as cyclosporine, vancomycin or cisplatin [3]. The mechanism of this wide-ranging protection entails blockade of cholesterol raft internalization by the cilastatin/DHP-I complex and decreased membrane turnover. As a result, nephrotoxic compound uptake is decreased. Furthermore, cilastatin blocks signalling by death receptors present in cholesterol rafts, such Fas, whose expression is increased during kidney injury, including bacterial lipopolysaccharide (LPS)-induced AKI and lung injury [4]. The cilastatin/DHP-I complex prevents internalization, trimerization and signalling through the Fas/Fas ligand complex [3]. Consequently, programmed cell death is prevented, and thus the amplifying inflammatory and oxidative wave of cell damage [3,5]. Kidney protection by cilastatin is also observed in rodent models of septic shock [6]. DHP-I is also expressed in lung alveolar epithelial cells. We have now observed for the first time that cilastatin blocks efficiently the development of histological lung injury associated with LPS-induced respiratory distress, decreasing inflammatory cell infiltration (Figure 1). Both severe sepsis and COVID-19 may be complicated by respiratory distress and AKI, which are associated with higher mortality [7]. In this regard, the cytokine storm elicited by LPS may mimic the cytokine storm of severe COVID-19.
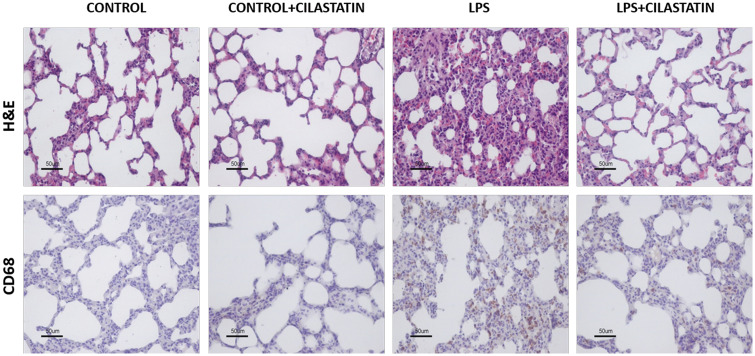
FIGURE 1:
Cilastatin improves bacterial LPS-induced lung injury in vivo. Thirty-six 6-week-old male Wistar rats weighing 150–200 g were administered intraperitoneally with 10 mg/kg LPS from Escherichia coli O55:B5 (Sigma Aldrich, St Louis, MO, USA) or its vehicle (0.9% NaCl) and 150 mg/kg cilastatin (Acs Dobfar, Tribiano, Milan, Italy) or its vehicle (0.9% NaCl) also injected intraperitoneally. The dose of cilastatin was administered just before the administration of LPS, and the rats (n = 9 animals per group) were euthanized within 24 h. Upper row: paraffin-embedded lung sections stained with haematoxylin–eosin (H&E). Control group shows normal lung structure; LPS-injected lungs show severe alveolar wall oedema, congestion, haemorrhage and increased inflammatory cell numbers. These changes were significantly reduced by treatment with cilastatin, with the lungs showing almost normal morphology. Lower row: immunolocalization of CD68 (monocyte/macrophage) in lung sections. Note increased CD68-stained cells in LPS-injected rats compared with the control group (staining score: 17.00 ± 3.50 versus 5.20 ± 1.16; P = 0.0014). Cilastatin significantly reduced the increase of CD68 expression induced by LPS (staining score: 5.50 ± 1.63 versus 17.00 ± 3.50; P = 0.0012). In both cases, treatment with cilastatin alone had no effects on lung morphology or inflammation.
Cilastatin is a fully developed parenteral drug that is clinically safe and currently approved for human use as a fixed combination with imipenem. Generic imipenem/cilastatin is already available. A dose-escalating Phase I clinical trial to evaluate the safety of higher doses of cilastatin than those authorized in the data sheet was completed in 2018 (NCT03595189). During the study, there were no clinically relevant alterations in the physical examination, vital signs and electrocardiogram recordings; minimal changes observed in haematological and biochemistry parameters had neither clinical relevance nor relationship with the study drug; there were no abnormalities detected in the Holter records performed during the study and there were no local tolerance warnings during the continuous administration of the drug.
Based on the preclinical evidence of kidney and lung protection in the context of endotoxemia as presented in this letter, we propose that cilastatin should be studied in clinical trials as a potential therapy for COVID-19. The current understanding of the drug mechanism of action suggests that it may both decrease viral entry and consequently viral replication, and additionally may protect from the deleterious consequences of the cytokine storm observed in severe COVID-19 (Figure 2). Indeed, a clinical trial of the Fas ligand trap asunercept is planned (https://www.pharmiweb.com/press-release/2020-07-28/apogenix-to-start-european-clinical-phase-ii-trial-with-asunercept-in-covid-19-patients).
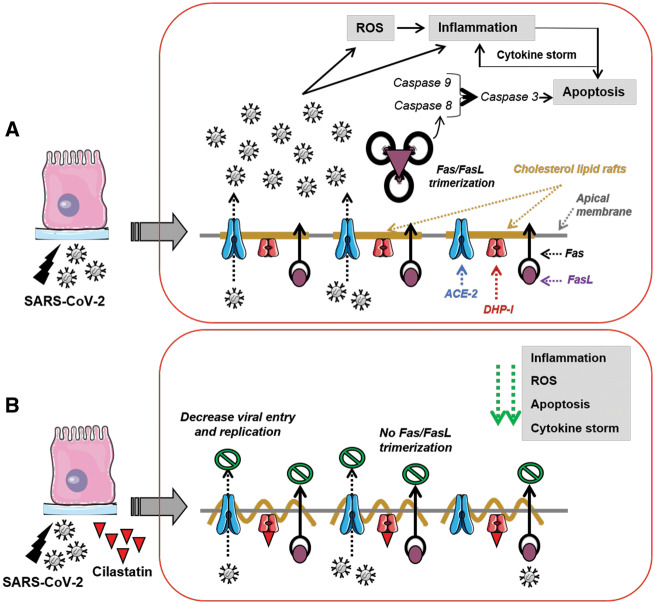
FIGURE 2:
Cilastatin may decrease SARS-CoV-2 replication and cell damage. (A) This represents SARS-CoV-2 infection of kidney/lung epithelium through ACE2 receptor, thus activating Fas/Fas ligand (FasL) trimerization and triggering reactive oxygen species (ROS), inflammation and apoptosis, leading to exponential cytokine storm, cell damage and death. (B) This shows cilastatin as a potential treatment that may prevent SARS-CoV-2 internalization and replication and protect from cell damage by blocking specifically DHP-I enzyme localized in cholesterol lipid rafts of the apical membrane, avoiding Fas/FasL trimerization and diminishing ROS, inflammation, apoptosis and cytokine storm.
FUNDING
The results of the cilastatin basic research were supported by Spanish grants from the Ministry of Economy and Competitiveness ISCIII-FIS (PI11/01132, PI14/01195 and PI17/00276 cofinanced by FEDER Funds from the European Commission, ‘A way of making Europe’), ISCIII-RETIC REDinREN/RD16/0009/0026, Comunidad de Madrid S2017-BMD-3686 (CIFRA2-CM) and Fundación Senefro (nephrology research grant SENEFRO 18/01). The data that support the findings presented in this letter are available from the corresponding author upon reasonable request. The Renal Physiopathology Laboratory dedicates this work to the memory of its founder, Dr Alberto Tejedor, inventor of cilastatin as a kidney protector, who died as a result of the dramatic effects of COVID-19.
AUTHORS’ CONTRIBUTIONS
M.Á.G.-N., C.G.-G. and V.A.P.-F. performed experiments, designed the figure and reviewed critically the final manuscript. A.L. designed the idea, did the bibliographic search and wrote the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
A.L. is a coinventor of cilastatin patents as a renal protector against toxic injuries (‘Use of cilastatin to reduce nephrotoxicity of various compounds’, patent numbers: EP 2143429 B1; US 9,216,185 B2; US 9,522,128 B2; US-9,757,349 B2). M.Á.G.-N. and A.L. are coinventors of the cilastatin patent for the treatment of sepsis and sepsis-associated AKI (‘Cilastatin for use in the treatment of sepsis’, Application PCT/EP2017/065609). All patents are assigned to Fundación para la Investigación Biomédica del Hospital Gregorio Marañón (FIBHGM). The rest of the authors declare no conflict of interest.
REFERENCES
- 1. Rubin S, Orieux A, Prevel R. et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J 2020; 13: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann M, Simon Schroeder H, Krüger N. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humanes B, Lazaro A, Camano S. et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int 2012; 82: 652–663 [DOI] [PubMed] [Google Scholar]
- 4. Ortiz-Arduan A, Danoff TM, Kalluri R. et al. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol 1996; 271: F1193– F1–201. [DOI] [PubMed] [Google Scholar]
- 5. Humanes B, Camaño S, Lara JM. et al. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol Dial Transplant 2017; 32: 1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazaro A, González-Nicolás MA, Humanes B. et al. Renal cholesterol rafts blockade ameliorates septic multiorganic failure. J Am Soc Nephrol 2017; 28: 189 [Google Scholar]
- 7. Li H, Liu L, Zhang D. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020; 395: 1517–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]