Abstract
An 84-year-old man with coronavirus disease 2019 pneumonia developed ST-segment-elevation myocardial infarction and was brought to the catheterization laboratory. His angiogram showed a haziness in distal right coronary artery, and optical coherence tomography (OCT) exhibited vascular spasm and OCT-defined plaque erosion, which were thought to be the causes of non-obstructive myocardial infarction.
<Learning objective: Severe acute respiratory syndrome coronavirus 2 infection provokes various complications, which include acute myocardial infarction (AMI). Nevertheless, the mechanisms and characteristics of AMI in patients with coronavirus disease 2019 have not been elucidated. In the present case, coronary spasm and optical coherence tomography (OCT)-defined plaque erosion were confirmed as the substrates of coronary thrombosis by the findings of intracoronary OCT.>
Keywords: COVID-19, Acute coronary syndrome, Plaque erosion, Optical coherence tomography
Introduction
Infectious diseases caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is called the coronavirus disease 2019 (COVID-19), have become worldwide threats to public health. Although the potential impact of COVID-19 on cardiovascular disease (CVD) has been reported [1], [2], the mechanisms or characteristics of acute coronary syndrome occurring in COVID-19 patients has not been elucidated. Of note, there is a paucity of reports on the detailed description of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19. We describe a case of STEMI which developed during the treatment for severe COVID-19 pneumonia, with detailed information on angiographical and optical coherence tomography (OCT) findings of the culprit lesion.
Case report
An 84-year-old man presented to a local hospital with a 1-day history of fever, chill, and dyspnea. The patient had histories of chronic obstructive pulmonary disease without medication or oxygen therapy and prostate cancer, which had been observed by his primary physician. He quit smoking 10 years before the admission after 54 years of smoking history. He did not have hypertension, dyslipidemia, diabetes, or family history of coronary artery disease.
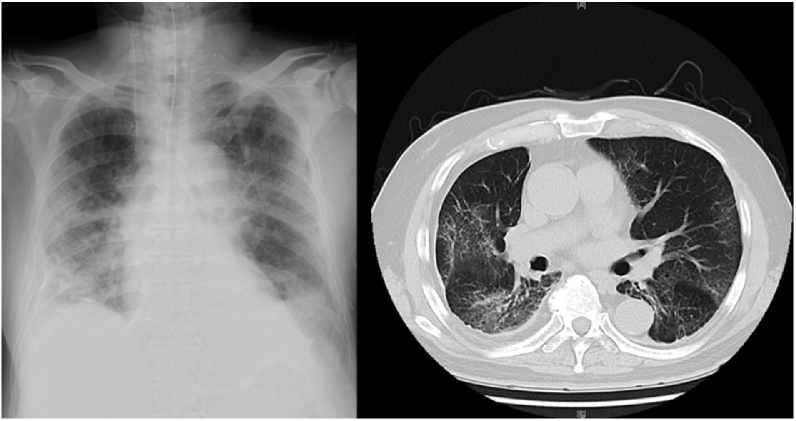
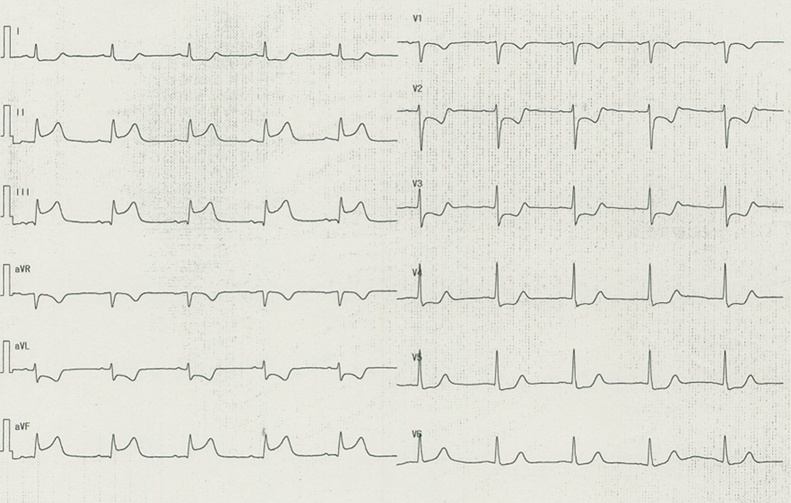
His initial chest computed tomography demonstrated bibasilar ground-glass opacities (Fig. 1) and his nasopharyngeal swab sample was positive for SARS-CoV-2 on real-time reverse transcriptase polymerase chain reaction assay. He was diagnosed with COVID-19 pneumonia and admitted to the hospital. Despite the medical therapy with oral favipiravir 1600 mg/day, severe hypoxemia was sustained. He was transferred to our hospital for further intensive care. On admission to our hospital, the patient was sedated and intubated, and mechanical ventilation was initiated because of progressive hypoxia. In consideration of hyperinflammatory status in severe COVID-19 pneumonia, immunosuppressive therapy with tocilizumab 600 mg/day was administered for 2 days. In the morning of the 3rd hospital day, the lead I on the continuous electrocardiogram (ECG) monitoring showed ST-segment depression without instability of hemodynamic or respiratory status. The 12-lead ECG revealed ST-segment elevation in the inferior leads (Fig. 2) and cardiologists were consulted.
Fig. 1.
Initial chest X-ray and chest computed tomography. The chest X-ray showed bibasilar opacities and computed tomography showed bibasilar-predominant ground-glass opacities.
Fig. 2.
Electrocardiogram on the 3rd hospital day. The electrocardiogram shows ST-segment elevation in leads II, III, and aVF with ST-segment depression in V1-5.
Investigations
The 12-lead ECG demonstrated ST elevation in the inferior leads (Fig. 2). Transthoracic echocardiogram showed a reduced wall motion in infero-posterior region with preserved left ventricular ejection fraction of approximately 50%. Levels of high-sensitive troponin-I and creatine kinase were 20,201 pg/ml (normal <24 pg/ml) and 1288 IU/l (normal <248 IU/l), respectively, at the time of the consultation.
Management
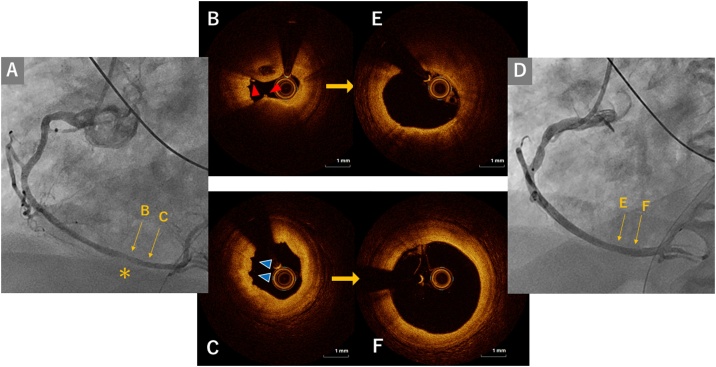
Given the sustained ST elevation and regional wall motion abnormality, he was brought to the catheterization laboratory for an emergent coronary angiography after pre-administration of aspirin 200 mg and a bolus injection of intravenous unfractionated heparin 3000 U. His angiograms revealed an intact left coronary artery. In the distal right coronary artery, mild stenosis with a hazy appearance of contrast opacification was identified (Fig. 3). Intracoronary OCT (Dragonfly Optis™, Abbott Vascular, Santa Clara, CA, USA) was performed to evaluate the lesion morphology, which exhibited a folded appearance of the intimal layer indicating spastic arteries [3] and intraluminal thrombi on a plaque with intact fibrous cap indicating OCT-defined plaque erosion [4]. After initial OCT examination, 200 μg of intracoronary isosorbide dinitrate was administered, which resolved diffuse vasospasm in the right coronary artery on angiograms and OCT (Fig. 3) and resulted in the rapid resolution of ST-segment elevation. These examinations confirmed the diagnosis of STEMI caused by coronary vasospasm and OCT-defined plaque erosion. Intravenous nicorandil followed by oral benidipine 4 mg was given for the prevention of coronary spasm after coronary angiography. In addition, continuous intravenous injection of unfractionated heparin was initiated against coronary thrombosis. Creatine kinase elevated to the peak level of 2336 IU/l at 5 h after catheterization. Left ventricular function was preserved with an ejection fraction of 50%. The treatment for COVID-19 pneumonia was continued without the recurrence of AMI.
Fig. 3.
Coronary angiograms and optical coherence tomography images in the culprit lesion before and after intracoronary injection of isosorbide dinitrate. (A) Coronary angiogram before coronary injection of isosorbide dinitrate (ISDN). In the distal segment of his right coronary artery, a haziness of contrast opacity is identified on angiogram before intracoronary injection of isosorbide dinitrate (A, yellow asterisk). Corresponding optical coherence tomography (OCT) images of the segment indicated on the angiograms are shown in the middle panel (B)(C)(E)(F). Intraluminal thrombi (B, red arrow) on a plaque with an intact fibrous cap indicating OCT-defined plaque erosion (B) and a folded appearance of the intimal layer indicating arterial spasm (C, blue arrows) were observed. (D) Coronary angiogram after ISDN. Coronary spasm was resolved both on angiogram and OCT (E, F).
Discussion
To the best of our knowledge, this is the first description of a case in which coronary spasm and OCT-defined erosion were confirmed on intracoronary OCT as the causes of STEMI occurring in a patient with COVID-19 pneumonia.
Since the pandemic of COVID-19, the number of patients presenting to hospitals with acute myocardial infarction (AMI) has been reported to have decreased [1], while the patients with COVID-19 were shown to have frequent myocardial injuries or infarctions during the treatment for COVID-19 [2]. Thus, the clinical presentation of AMI may be different in the condition of SARS-CoV-2 infection from those in the normal condition. Nevertheless, the mechanisms and characteristics of AMI in patients with COVID-19 have not been elucidated except an anecdote that more frequent AMI are attributable to non-obstructive coronary arteries than usual [5]. In the present case, coronary spasm and OCT-defined plaque erosion were confirmed as the substrates of coronary thrombosis by the findings of intracoronary OCT.
Coronary spasm is thought to represent a deteriorated endothelial function of coronary arteries [6]. Endothelial injuries in patients with COVID-19 have been reported in pathological investigations [7], suggesting a potential involvement of angiotensin-converting enzyme 2 receptor, which may result in the impairment of the endothelial function of coronary arteries. In addition to the intrinsic endothelial function, it has been reported that chemical mediators may play significant roles in the mechanism of coronary spasm. For example, in patients with anaphylactic syndrome, spastic angina frequently occurs due to the excessive release of inflammatory mediators induced by allergic reaction, which is known as Kounis syndrome. Same as anaphylactic status, patients with COVID-19 are shown to have increased levels of inflammatory cytokines, which deteriorate the systemic conditions leading to acute respiratory distress syndrome or multi-organ failure [8]. As well as the respiratory system, coronary arteries may also be influenced by the inflammatory mediators released in the patients with COVID-19, which potentially induce coronary spasm as shown in the present case. Plaque erosion is the second major mechanism of AMI following plaque rupture [4], [9]. Denudation of endothelial layer of coronary arteries complicated with a thrombogenic status of blood results in intracoronary thrombus. Endothelial injuries can be induced by COVID-19 as described above [7], which may expose the subendothelial layer and provide a thrombogenic status on the surface of coronary arteries. Moreover, the COVID-19 reportedly induces systemic coagulopathy, which leads to pulmonary embolization, venous thrombosis, embolic stroke [10], and potentially the susceptibility to coronary thrombosis. In the present case, causal relationship between COVID-19 and coronary thrombosis could not be proven. Nevertheless, considering these potential mechanisms, coronary spasm and plaque erosion might be prevalent pathogeneses of AMI in COVID-19 patients.
Conclusions
Coronary vasospasm and plaque erosion were potential substrates of STEMI in the current COVID-19 patient. Those mechanisms may be accelerated by COVID-19-induced hyperinflammatory and hypercoagulable conditions.
Disclosures
The authors declare that there is no conflict of interest regarding this manuscript.
Sources of funding
None.
Acknowledgments
None.
References
- 1.Solomon M.D., McNulty E.J., Rana J.S., Leong T.K., Lee C., Sung S.-H. The covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 2.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka A., Shimada K., Tearney G.J., Kitabata H., Taguchi H., Fukuda S. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011;58:1608–1613. doi: 10.1016/j.jacc.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia H., Abtahian F., Aguirre A.D., Lee S., Chia S., Lowe H. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B. ST-segment elevation in patients with covid-19 – a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kugiyama K., Yasue H., Okumura K., Ogawa H., Fujimoto K., Nakao K. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–271. doi: 10.1161/01.cir.94.3.266. [DOI] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. Lessons from sudden coronary death. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M.J.H.A., Van Der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]