Abstract
The theta rhythm during waking has been associated with voluntary motor activity and learning processes involving the hippocampus. Theta also occurs continuously during rapid eye movement (REM) sleep where it likely serves memory consolidation. Theta amplitude builds across wakefulness and is the best indicator of the homeostatic need for non-REM (NREM) sleep. Although REM sleep is homeostatically regulated independently of NREM sleep, the drivers of REM sleep regulation are under debate. The dynamics of theta within REM sleep bouts have not been thoroughly explored. We equipped 20 male rats with sleep instrumentation and hippocampal electrodes to measure theta across normal sleep/waking periods over the first 4 h of the sleep phase on two consecutive days. We found that theta power decreased by a third, on average, within individual REM sleep bouts, but recovered between bouts. Thus, there was no general decline in theta power across the duration of the recording period or between days. The time constant of theta power decline within a REM sleep bout was the same whether the bout was short, midlength, or long, and did not predict the behavioral state immediately following the REM sleep bout. Interestingly, the more time spent in NREM sleep prior to REM sleep, the larger the decline in theta power during REM sleep, indicating that REM sleep theta may be homeostatically driven by NREM sleep just as NREM delta power is driven by the length of prior waking and by waking theta. Potential causes and implications for this phenomenon are discussed.
Keywords: Theta, REM sleep, Rat, Hippocampus, Phasic activity, Learning
Introduction
Theta is a 5–9 Hz oscillation in neuronal membrane potential voltage that can be recorded from a number of cortical sites in the electroencephalogram (EEG), but is most commonly recorded from the hippocampus. The theta oscillation is set in motion by acetylcholine and/or GABA from the medial septal nucleus. Theta amplitude reflects the number of individual neurons deflecting their membrane potential at that rhythm in synchrony. Theta power over a window of time is influenced by both the amplitude of the signal and its duration across the window.
Theta is a functionally important brain rhythm that has been shown to be critical to learning and memory processes during waking (Winson 1978; Mitchell, Rawlins et al. 1982; Leutgeb and Mizumori 1999; Fletcher, Calhoun et al. 2006). In addition, theta during REM sleep is essential to hippocampus dependent memory consolidation (Boyce et al., 2016). Theta is a defining feature of REM sleep. Indeed, the same brainstem regions that control REM sleep also regulate theta (Vertes, 1981; Kitchigina et al., 1999). Theta during REM sleep increases following training (Fogel, Smith et al. 2007) implying a homeostatic mechanism driving its occurrence. Delta frequency (0.5–4 Hz) power, which dominates NREM sleep, is often used as a surrogate for the drive for sleep: the longer and more intense the waking experience, the larger the delta power at the beginning of each sleep period (Vyazovskiy and Tobler 2005). Although REM sleep is homeostatically regulated independent of NREM sleep, we wondered whether its dominant frequency, theta, would show a similar dynamic as delta, that could indicate the status of the REM sleep homeostatic drive.
We sought to more fully characterize theta activity during REM sleep, as variations in theta power could both be significant for learning and memory consolidation and be a biomarker indicating a homeostatic regulator of REM sleep. Here we describe the dynamics of theta power within REM sleep bouts: whether theta power was influenced by the length of the REM sleep bout, by the density of REM sleep bouts, by the interruption of other states, by the identity of the state following REM sleep and finally, by the length of any prior state. We interpret these finding in light of a homeostatic controller for REM and discuss the implications of theta dynamics for the function of REM sleep.
Methods
Subjects
Twenty male Fischer 344 rats (Simonsen Labs, Gilroy, CA) were used. Animals were housed individually in standard rodent cages that were located within an environmentally regulated chamber. Temperature was maintained at 23ºC with a 12:12 light cycle. Fresh rat chow mush (50% pellet, 50% water mix) was provided daily and water was available ad libitum. Prior to surgery, animals were pre-screened for visual acuity in the Morris Water Maze for a separate experiment performed later on the same animals. At least 2 days following visual screening, animals were surgically implanted with instrumentation to perform sleep/waking recordings. All procedures were approved by the University of Michigan’s University Committee on Use and Care of Animals in accordance with the United States Department of Agriculture Animal Welfare Act and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Surgical implantation of electrodes for sleep/waking analysis
Animals were anesthetized with sodium pentobarbital and placed into a stereotaxic frame. An incision was made on the top of the skull and the skin was retracted. After cleaning the surface of the skull, 4 holes were drilled through the cranium and self-tapping screw electrodes were inserted. Two electrodes were placed bilaterally over the dorsal hippocampus (2.5 mm lateral to midline, 3.5 mm posterior to bregma, 2.0 mm ventral) and two electrodes were placed bilaterally over the frontal area (2.5 mm lateral to midline, 1.5 mm anterior to bregma, 1.5 mm ventral) for electroencephalographic (EEG) recordings. Two flexible wire electrodes were threaded through the dorsal neck muscles for electromyographic (EMG) recordings. Gold amphenol pins were connected to the ends of each electrode then placed into a six pin connector (Plastics One, Roanoke, VA) which was attached to the skull by dental acrylic. Animals were given Tylenol in their drinking water two days prior to surgery, orally following surgery, and 3 days postoperatively. All animals were given at least 7 days to recover from surgery prior to beginning the experiment.
Sleep recording and analysis — surface EEG recordings
Following recovery from surgery, animals were connected to the recording system via a lightweight, flexible tether that was attached to a commutator, allowing relatively free movement within their home cage. We used the AD recording system (MA Wilson, L Frank, M.I.T., Cambridge, MA) to sample signals at 1000 samples/second, filtered the signal between 0.1–100 Hz using a second order Butterworth filter, and then amplified the signal. Prior to analysis, signals were down-sampled to 250 samples/second by taking every 4th data point. The best combination of hippocampal EEG referenced to frontal EEG was used for one channel of EEG recording, while the two EMGs were referenced to each other for one channel of EMG recording. Animals were given 2 days to acclimate to the tethers prior to baseline recordings. On each acclimation and baseline day, animals were given fresh soft chow (standard pellets dissolved in water) shortly after the beginning of the light phase. After feeding, animals were not disturbed at any time during the day. Following acclimation, sleep/waking activity was analyzed for 4 hours on each of 2 consecutive days, beginning within 2 hours of lights on in the light phase of the 12/12 light dark cycle, when sleep is predominant in nocturnal animals like the rat. The majority of sleep and learning studies on rats present the learning session within the first hour or two after lights on. Over the past three decades, studies timed to the early part of the light period all find that REM sleep occurring in the first 4–6 hours after learning constitutes a “critical window” for memory consolidation (Smith and Rose, 1996; Legault et al., 2004; Bjorness et al., 2005). Therefore, we chose the first half of the sleep phase because we wanted to examine the dynamics of REM sleep occurring within this particular window of time.
After the baseline recording was completed, the data were transferred from the recording PC, stored onto disk, and scored for sleep/waking state off-line using custom Matlab (Mathworks, Natick, MA) programs. Briefly, EEG and EMG files were read into Sleep Scorer (Gross et al., 2009), an open-source stage scoring software in which a human scorer created a training file with a few bouts of each state manually assigned. Next, the scorer used the training file to set thresholds in another program (Auto-Scorer, Gross et al., 2009) that bins the data in 10-sec epochs then uses those thresholds and logic to automatically assign one of five sleep/waking states to each epoch. Finally, the automatically scored file was reviewed and corrected by a human scorer—with every epoch assigned the state that occupied the majority of the epoch.
Active waking (AW) consists of visible theta activity and high EMG activity, Quiet waking (QW) consists of low amplitude, desynchronized EEG and relatively little EMG activity, NREM sleep consists of high amplitude, synchronized EEG and low EMG activity, Transition-to-REM sleep (TR) consists of high amplitude spindle activity and low EMG activity, and REM sleep consists of clear, sustained theta activity and phasic muscle twitches on a background of low EMG. A REM sleep bout was defined as beginning with two or more consecutive epochs of REM sleep and terminating with three consecutive epochs without REM sleep, i.e. intrusions of 10–20 sec of another state are included within the REM sleep bout, while 30 contiguous seconds or more of any state other than REM sleep signaled the end of the REM sleep bout. Following state assignment, theta power during REM sleep was further analyzed.
Theta power analysis
Power in the 5–9 Hz theta frequency band of the hippocampal EEG signal was calculated for each 10-sec epoch of REM sleep in MATLAB using built-in functions. Briefly, each 10-sec epoch of EEG data was multiplied by a Hann window of the same length, resulting in a Hann tapered signal, xh. The power spectrum (Sxx) was calculated using only the real values from the following equation shown in MATLAB (Mathworks, Natick, MA) syntax:
where the MATLAB function ‘real’ extracts only the real values of an array, ‘fft’ calculates the fast Fourier transform of an array, and ‘conj’ calculates the complex conjugate of an array. The temporal resolution, dt, is equal to the inverse of the down-sampled sampling rate (i.e. 1/250 = 0.004 sec). The frequency resolution, df, is equal to the inverse of the epoch duration (i.e. 1/10 = 0.1 Hz). The resulting power spectrum is given for frequencies up to the low pass frequency of the signal (i.e. 100 Hz). Finally, the theta band power for each epoch was then calculated using a rectangular approximation of the integral of the power spectrum curve from 5 to 9 Hz. In the case of data presented in the results section Theta power by one Hz bins (5–9 Hz), the theta band power was calculated in the same way, for each individual nonoverlapping 1 Hz band (5.0–5.9 Hz, 6,0–6.9 Hz, 7.0–7.9 Hz, 8.0–8.9 Hz, and 9.0–9.9 Hz).
All REM sleep bouts longer than 10 sec were used in the analysis of theta power. EEG theta power was compared across up to five different epochs, spread across the REM sleep period. These five epochs analyzed were from the beginning (first and second epoch), middle, and end (last and second-to-last epoch) of each REM sleep bout. Wherever possible (i.e., in REM sleep bouts at least 70 seconds long), the calculations moved away from the first and last epochs to maximize the probability that the entire length of the first and last epochs used was in REM sleep rather than including some of the state to/from which REM transitioned.
When we analyzed theta power in 1 Hz bins, we calculated the power of each individual frequency across the five points of each REM sleep bout. To calculate EMG power, the signal was rectified and integrated.
Statistical analysis
SPSS (SPSS Inc., Chicago, IL) and Microsoft Excel (Microsoft Corporation, Redmond, WA) were used for statistical comparisons. Prior to statistical analysis, each REM sleep bout was categorized for length (short, medium, long), single/sequential status (single, sequential), interruption within ongoing REM (with interruption, without interruption), and subsequent state (active waking, quiet waking, NREM sleep) such that each REM sleep bout was assigned a category for each of the listed parameters. A repeated measures ANOVA with General Linear Model and within-subject design was used to compare effects between all 5 time points (dependent factors) averaged across REM sleep bouts within the REM sleep bout subcategory, with a Huynh-Feldt adjustment when variability across time points was unequal. Separate repeated measures ANOVA analyses were run for each REM sleep bout category (i.e., by length, single/sequential, with and without interruption, subsequent state; characterization for each designation described within results section).
A repeated measures ANOVA with General Linear Model and within-subject design was used to compare effects (REM sleep bout characteristics such as length and single vs. sequential) between all 5 time points (dependent factors) averaged across REM sleep bouts, with a Huynh-Feldt adjustment when variability across time points was unequal. FFT values were first averaged within an animal then averaged across animals. A mixed model design with repeated measures was used to compare theta power between all 5 time points when all REM sleep bouts were considered (SPSS script by animal, number of REM sleep bouts, and day with the number of epochs within the REM bout). Paired and two sample t-tests were used for comparisons of values averaged within rats. These include comparisons of percent change in theta power between the first and last epochs of REM sleep across the various REM sleep bout divisions (e.g. single vs sequential, interrupted vs contiguous, etc.) and comparison of average duration of REM sleep bouts across divisions. Significance level for all tests was set at p ≤ 0.05.
Results
Theta power decreased within individual REM sleep bouts
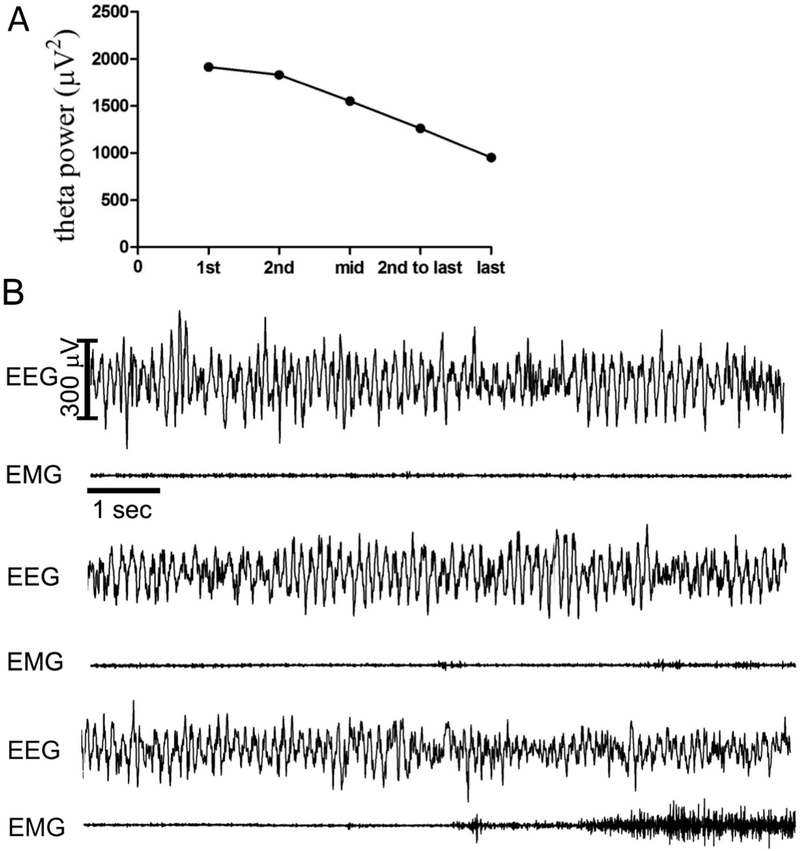
An example of theta decrease within a REM sleep bout is shown in Figure 1A, along with raw EEG and EMG traces recorded during that bout (Figure 1B). Average theta power decreased significantly within REM sleep bouts (F with Huynh-Feldt adjustment [3.236, 61.478] = 21.767, p<0.001) with theta in the 1st and 2nd epochs of the REM sleep bout higher than at the other 3 time points within REM sleep (middle, 2nd-to-last, and last epochs, Figure 2A). Theta power decreased from the beginning of a REM sleep bout to the middle epoch of the REM sleep bout by approximately 21%, however the decrease from the middle epoch to the last epoch was only approximately 11% (n.s.). When all REM sleep bouts (rather than the average for each animal) were compared using a mixed model with repeated measures design, theta power decreased similarly as in the averaged REM sleep bout comparison (Table 1). The only difference between average theta power (Figure 2A) vs. all bouts with repeated measures (Table 1) was higher theta power in the 1st compared to the 2nd epoch when all bouts were used (p<0.05). While the decrease in average theta power within REM sleep bouts was significant, there was a considerable amount of variability as can be seen from the standard deviation and standard error values given in Table 1.
Fig. 1. –
Example of power in the 5–9 Hz theta band across a REM sleep bout and the electroencephalogram activity. A. Example of theta power at five 10-sec epochs within a REM sleep bout. B. Ten second epochs of EEG and EMG traces corresponding to the 1st epoch of REM sleep (top set of traces), middle epoch of REM sleep (middle set), and the last epoch of REM sleep (bottom set) from the example given above.
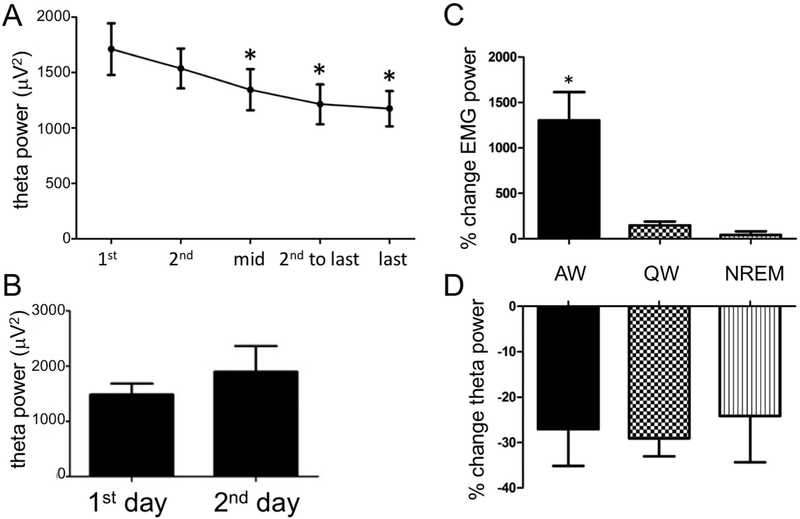
Fig. 2. –
A. Decrease in average theta power within REM sleep bouts. Theta power decreases from the initial two epochs of REM sleep bouts to the mid and last 2 epochs (N=20 rats, total of 419 REM sleep bouts. Significant differences from the first epoch are denoted by asterisk, p≤0.01). B. Average theta power in the first epoch of the first REM sleep bout on day 1 versus the first epoch of the last REM sleep bout on day 2. There is no significant change in theta power across the recording period. C. Percent increase in EMG power from the last epoch of REM sleep to the following epoch of active waking (AW), quiet waking (QW), and NREM sleep. EMG power increased significantly between the last REM sleep epoch to the epoch following the REM sleep episode only when REM sleep was followed by active waking (significant change from REM sleep denoted by asterisk, p<0.001). D. Percent decrease in theta power within REM sleep bouts preceding AW, QW, and NREM sleep. Theta power decreases significantly and similarly from the 1st epoch to the last epoch of REM sleep no matter what state follows REM sleep.
Tab. 1 –
Theta power averaged across all 419 REM sleep bouts at 5 points within each REM sleep bout, with standard deviation and standard error.
| average (μV2) | standard deviation | standard error | |
|---|---|---|---|
| 1st epoch | 1758.4 | 1397.7 | 320.6 |
| 2nd epoch | 1513.8 | 989.0 | 226.9 |
| middle epoch | 1312.4 | 941.7 | 216.0 |
| 2nd-to-last epoch | 1216.6 | 991.1 | 227.4 |
| last epoch | 1201.1 | 962.9 | 220.9 |
To ensure that the decrease in theta power within REM sleep bouts was not due to an overall decrease in theta power across time (e.g., due to a decline in signal quality), theta power in the 1st epoch of the first REM sleep bout on the first day of recording was compared to theta power in the first epoch of the last REM sleep bout on the second day of recording. There was no significant difference in theta power in the 1st epoch of REM sleep across days (Figure 2 B). Additionally, percent decrease in theta power within REM sleep bouts was consistent across time. There was no difference in percent decrease in theta power in the 1st REM sleep bout on each day compared to percent decrease in theta power on the last REM sleep bout on each day (data not shown).
Theta power decreased as EMG power increased within REM sleep
Since theta power is greater during phasic activities such as eye movements, PGO waves, and muscle twitches (Sano, Iwahara et al. 1973; Whishaw and Vanderwolf 1973; Robinson, Kramis et al. 1977; Karashima, Nakao et al. 2004), EMG power reflecting such phasic twitches was analyzed within REM sleep bouts. In addition to EMG power in the five analyzed epochs within each REM sleep bout, EMG power in the epoch following REM sleep was also compared since EMG power would be expected to be higher immediately following REM sleep, particularly in active waking. This increase in EMG power could serve as a positive control for accuracy of scoring transitions from REM sleep to another state, lest the appearance of that subsequent state unduly influence our theta power calculations. Indeed, as expected, there was an increase in EMG power between the REM sleep bouts and the epoch following REM sleep (F[5,95] = 3.898, p<0.05). This EMG power change was due to the epoch following REM sleep since there was no difference in EMG power between any of the 5 epochs during REM sleep. The increase in EMG power was largest when active waking followed REM sleep (F[1.012,15.173]=13.001, p<0.01, Figure 2C).
Theta power decreased regardless of subsequent state
The next factor investigated was whether the state following REM sleep affected the decline in theta power, i.e., is there a difference in theta power change when REM sleep is followed by active waking vs. quiet waking vs. NREM sleep? Theta power decreased significantly within REM sleep bouts that preceded active waking (F[4,60]=10.355, p<0.001, n=17 rats, 15.95% of bouts), quiet waking (F[3.116,60.156]=15.067, p<0.001, 63.33% of bouts), and NREM sleep (F[3.034,45.503]=5.894, p<0.01, n=17 rats, 20.71% of bouts), with some differences between them (Table 4). There was no difference in percent change in theta power from the 1st to last epoch of REM sleep between those REM sleep bouts that preceded active waking, quiet waking, and NREM sleep (Figure 2D). Also, there was no difference in average duration of REM sleep bouts preceding active waking, quiet waking, or NREM sleep (ave. REM sleep duration before: AW 132.3 +/− 12.5 sec, QW 129.2 +/− 10.1 sec, NREM 132.2 +/− 13.9 sec).
Tab. 4 -.
Theta power in REM sleep preceding active waking, quiet waking, and NREM sleep.
| AW | QW | NREM | |
|---|---|---|---|
| 1st epoch | 1725+/−261.7 | 1738.7+/−248 | 1545.9+/−241.2 |
| 2nd epoch | 1500.1+/−188.4 | 1561.3+/−194.2 | 1489.2+/−177.5 |
| middle epoch | 1300.1+/−221.1* | 1348.8+/−194.7*# | 1408.5+/−230.6* |
| 2nd-to-last epoch | 1142.3+/−172.5*# | 1251.6+/−193.7*# | 1180.9+/−224.8* |
| last epoch | 1128.6+/−144.8*# | 1220.1+/−175.9*# | 1055+/−148.3*# |
Average theta power (μV2) +/− standard error
indicates significant difference compared to the 1st epoch
indicates significant difference compared to the 2nd epoch
A minority of REM sleep bouts increased theta power
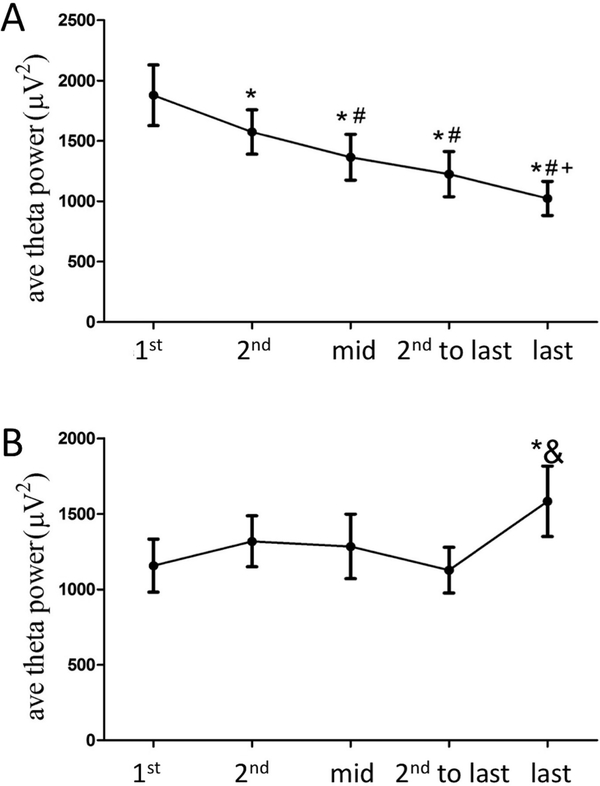
Upon closer inspection, it was evident that while most (73.4%) of the REM sleep periods resulted in a decrease in theta power, there was a proportion (26.6%) in which theta increased throughout the REM sleep bout (Figure 3). There were no instances of unchanged theta power. Thus, we divided REM sleep bouts that decreased from those that increased theta power for further inspection. For bouts of REM sleep with decreasing theta power (Figure 3A) it was found that theta power in the 1st epoch was greater than the 2nd epoch, and both were greater than the remaining epochs. The mid and 2nd to last epoch were not significantly different from each other, but were both greater than the last epoch (1st>2nd>mid=2nd-to-last>last, F with Huynh-Feldt adjustment [2.815,53.487] = 34.782, p<0.001). For the 26.6% of REM sleep bouts in which theta power increased (Figure 3B), the only significant difference in theta power was a significant increase in theta in the last epoch compared to the 1st and 2nd to last epochs (F with Huynh-Feldt adjustment [2.430,46.161] = 4.762, p<0.01).
Fig. 3 –
A. The majority of REM sleep bouts show a pattern of decreasing theta power from the early epochs to the later epochs (*=different from 1st epoch, #=different from 2nd epoch, +=different from mid epoch); B. 26.6% of REM sleep bouts show significantly higher theta power in the last epoch compared to the first (*) and 2nd to last (&) epochs (p<0.01).
Theta power by one Hz bins (5–9 Hz)
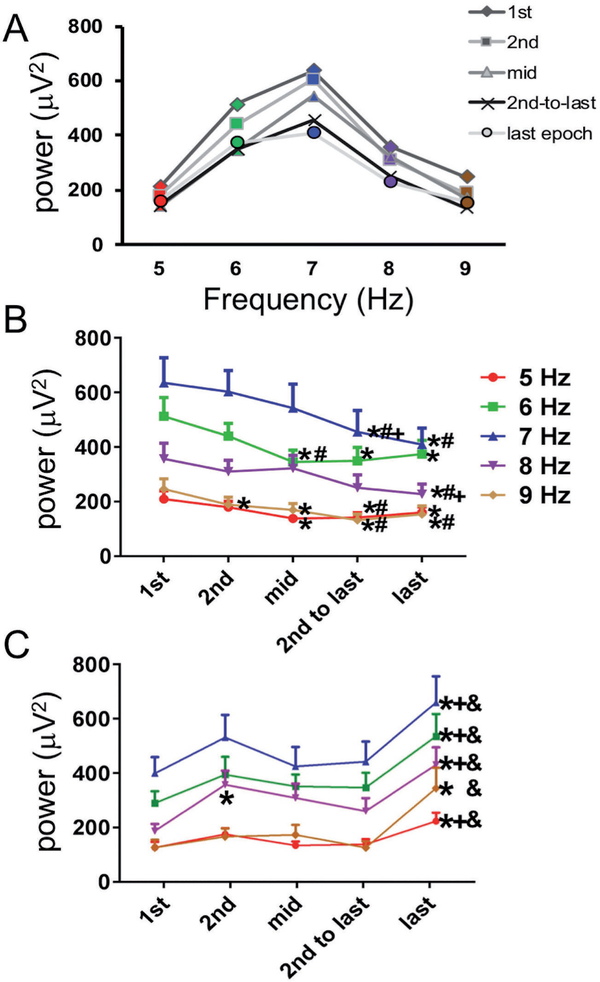
In order to determine if there was a difference in how much individual 1 Hz frequencies within the 5–9 Hz band contributed to the total theta power, power in each of the 5, 6, 7, 8, and 9 Hz bands was calculated separately. As expected, all bands showed a significant decrease in power from the 1st to last epoch of REM sleep. However, the average power of the 1st epoch was significantly different between bands (F[4, 76]=29.353, p<0.001) with power in the 5 Hz and 9 Hz bands significantly lower than all other bands (5 Hz ave = 209.9 +/− 29.4 μv2, 6 Hz ave = 512.8 +/− 69.2 μv2, 7 Hz ave = 635.6 +/− 91.9 μv2, 8 Hz ave 356.9 +/− 57.6 μv2, 9 Hz ave = 246.2 +/− 37.3 μv2, p<0.001). Power in the 6 and 7 Hz bands was greater than all other bands, but not significantly different from each other (Figure 4A). Next, percent change in power from 1st to last epoch was calculated. There was a significant difference in percent change in power between bands (F[4, 76]=3.716, p<0.01), with the 9 Hz band showing the greatest percent decline across REM sleep at 41.4 +/− 4.7%. When episodes were divided by whether theta power decreased (Figure 4B) or increased (Figure 4C) across an episode, the same general effects were found with the individual 5, 6, 7, 8, and 9 Hz bands as was found in the total theta band power.
Fig. 4. –
The individual bands that comprise the theta band (5, 6, 7, 8, and 9 Hz) show a similar pattern of decreasing power from the initial epochs of the REM sleep bout compared the later epochs of same REM sleep bout. Panels A and B are calculated from the 73.4% of REM sleep bouts with decreasing power. The 23.6% of REM sleep bouts that showed increasing power increased from the initial or 2nd to last epoch to the last epoch of the REM sleep bout (C). Symbols next to data points mean the time point is significantly different from: * 1st epoch, # 2nd epoch, + middle epoch, & 2nd-to-last epoch.
Theta power decreased in both short and long REM sleep bouts
Since REM sleep bouts shorter than 50 sec did not contribute to all of the 5 epochs, they could potentially skew the overall average towards the beginning and end points. Thus, we separately investigated whether REM sleep duration influenced the pattern of theta power across the 5 considered epochs. REM sleep bouts were divided into relatively short (≤110 sec), midlength (120–250 sec), and long durations (>250 sec). Table 2 shows that theta power decreased significantly within all three REM sleep bout lengths considered: short (F[4, 76]=8.863, p<0.001), midlength (F[3.055, 58.041]=17.994, p<0.001), and long (F[3.06, 33.662]=5.219, p<0.01). All three divisions showed similar patterns of decline in average theta power across REM sleep (Figure 5).
Tab. 2 –
Theta power within REM sleep bouts of different lengths.
| short | midlength | long | |
|---|---|---|---|
| 1st epoch | 1634.9+/−241.3 | 1823.9+/−246.7 | 1577.4+/−265.4 |
| 2nd epoch | 1450.9+/−190.1 | 1596.1+/−191* | 1603.2+/−274.9 |
| middle epoch | 1224+/−178*# | 1431.3+/−214.7* | 1323.5+/−264 |
| 2nd-to-last epoch | 1238.9+/−177.4* | 1184.3+/−180.1*#+ | 1128.8+/−394 |
| last epoch | 1248.2+/−181.2* | 1090.1+/−140.5*#+ | 891.4+/−158.5*# |
Average theta power (μV2) +/− standard error
indicates significant difference compared to the 1st epoch
indicates significant difference compared to the 2nd epoch
indicates significant difference compared to the midpoint epoch
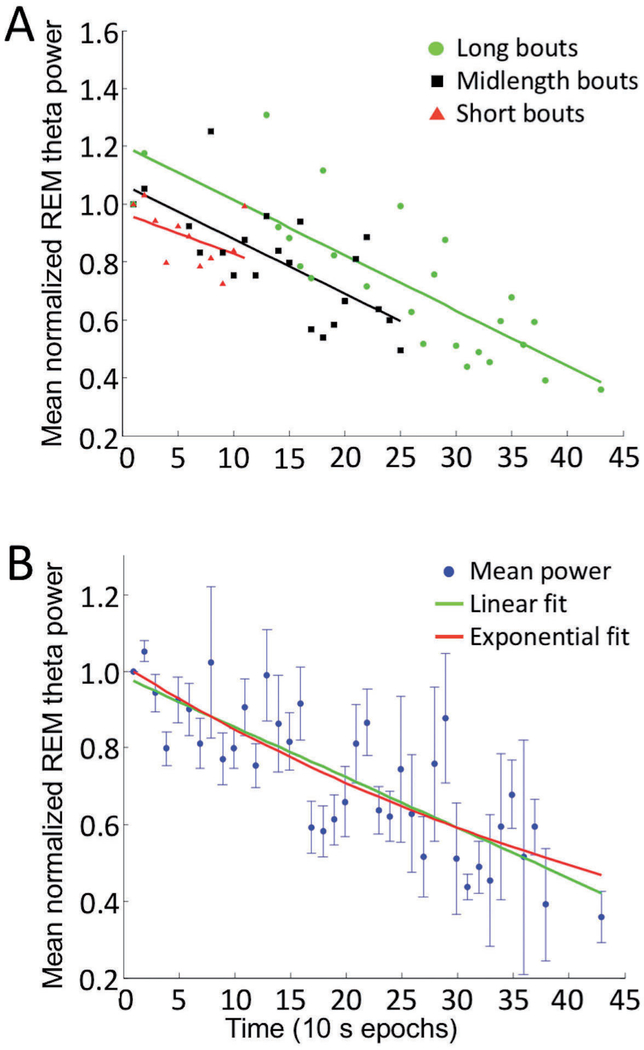
Fig. 5. –
Fit of theta power (normalized to the initial epoch) over time in all REM sleep bouts (A) parsed by length into short, midlength, or long bouts. Linear fit lines corresponding to each bout length are shown in the same color as the group. (B) Theta power normalized to the first epoch and averaged from all REM sleep bouts at each time point was fit by linear (green) and exponential (red) curves. Overall, theta power decreases across the bout can be fit equally well by linear and exponential curves.
Theta power decreased at a consistent rate over time
To better analyze the influence of time on theta power within a REM sleep bout, we normalized power values in each bout relative to theta power in its 1st epoch, thereby also reducing the influence of theta power variations between bouts. For this analysis, we omitted 9 REM sleep bouts whose theta power values exceeded mean values by 2 standard deviations. All bouts contributed to the average power at 10 and 20 sec since the shortest REM sleep bout considered was 20 sec long, but the number of power values contributing to the averages at other time points varied due to differences in REM sleep bout lengths. For example, the shortest REM sleep bout considered as part of the long bout group was 260 sec. Thus, the mid-bout epoch of the long bout group occurred at 130 sec or more into the REM sleep period. Accordingly, the long-bout group did not contribute to the averages from 30 s to 120 s (10 s epochs 3–12) in Figure 5B. When bouts were divided according to length, theta power decreased at similar linear rates for each length category (Figure 5A, short bouts: slope = −0.014, R-square = 0.13; midlength bouts: slope = −0.019, R-square = 0.47; long bouts: slope = −0.019, R-square = 0.66). All fits were computed using the curve fitting toolbox in MATLAB. The theta power decline over time across all REM sleep bouts could be fit equally well with a linear function (−0.013 t +0.987, R-square = 0.65) or a single exponential function (1.02 exp(−0.018 t), R-square = 0.64) (Figure 5B). These results suggest that theta power decreases at a consistent rate over time during REM sleep, regardless of bout duration.
Theta power decreased in both single and sequential REM sleep bouts
Other REM sleep characteristics may affect theta power. REM sleep bouts can occur either in isolation (single) or in clusters (sequential) (Amici, Zamboni et al. 1994; Zamboni, Perez et al. 1999). Single bouts were defined as having > 3 min of intervening NREM sleep or waking between them, while sequential bouts have ≤ 3 min of intervening waking or NREM sleep. Single and sequential REM sleep bouts are hypothesized to be functionally different and are differently affected by REM sleep deprivation (Amici, Zamboni et al. 1994; Zamboni, Perez et al. 1999; Zamboni, Amici et al. 2001). On average, single bouts accounted for 68.8% of all REM sleep bouts over the two recording days. Single and sequential bouts both showed a significant decrease in theta power (single F[2.514,47.774]=21.364, sequential F[4,72]=9.644, p<0.001). Single and sequential bouts had a similar pattern of theta power decline, with theta in the first two epochs significantly higher than the mid, 2nd to last, and last epochs. However, in single episodes, theta in the mid epochs was significantly higher than the 2nd to last and last epochs, while in sequential episodes there was no difference in power between the mid, 2nd to last, and last epochs. There was no significant difference in percent decrease in theta power between single and sequential bouts (single bouts, 33 +/− 3.2%, sequential bouts, 28.1 +/− 5.2%). Single bouts were significantly longer than sequential bouts (single ave. = 139.58 +/− 10.1 sec, sequential ave. = 95.05 +/− 9 sec, p<0.01, n=19 as one animal had no sequential bouts).
Theta power decreased with and without interruption
REM sleep is often interrupted in rats (Trachsel, Tobler et al. 1991). When REM sleep precedes and follows a short period, around 30 sec or less for rats, of another state, the entire time is often scored as one REM sleep bout (Webb and Dreblow 1982; Trachsel, Tobler et al. 1991; Benington, Kodali et al. 1994; Vivaldi, Ocampo et al. 1994). We tested whether short periods of an intervening state within a REM sleep bout affected REM sleep theta power. REM sleep bouts were divided into those that had 10–20 sec of an intervening state, usually NREM sleep, and those that had continuous REM sleep throughout. Interrupted REM sleep bouts accounted for 26.5% of all REM sleep bouts. Theta power decreased within REM sleep bouts that were interrupted (F[2.853,56.631]=14.635, p<0.001) as well as across non-interrupted bouts (F[4,76]=17.006, p<0.001, Table 3). Interrupted vs. non-interrupted REM sleep bouts were no different in percent change in theta power from the 1st to the last epoch of REM sleep.
Tab. 3 –
Theta power within interrupted and non-interrupted REM sleep bouts.
| interrupted | non-interrupted | |
|---|---|---|
| 1st epoch | 1697.9+/−233.3 | 1705.9+/−241.3 |
| 2nd epoch | 1551.6+/−190.6 | 1520.2+/−177.9 |
| middle epoch | 1290.8+/−191.4* | 1364.5+/−191.9*# |
| 2nd-to-last epoch | 1097.7+/−183.4*# | 1246.8+/−182.1*# |
| last epoch | 1061.9+/−142.2*#+ | 1210.4+/−174.3*# |
Average theta power (μV2) +/− standard error
indicates significant difference compared to the 1st epoch
indicates significant difference compared to the 2nd epoch
indicates significant difference compared to the midpoint epoch
Homeostatic influence on theta power within REM sleep
Finally, to determine if there was a homeostatic component to the dynamics of theta power during REM sleep bouts, time between successive REM sleep bouts was correlated with percent change in theta power within REM sleep. Amount of time in NREM sleep was significantly related to the change in theta power (p<0.05), with more NREM sleep linked to larger decreases in theta power within REM sleep. Total time between successive REM sleep bouts or time in any other state did not correlate with percent change in theta power, including prior time in REM sleep or time relative to the beginning of the light phase. Only time in NREM sleep predicted theta power declines across a REM sleep bout.
Discussion
In this study, we investigated the dynamics of theta activity within REM sleep bouts. Theta power declined within REM sleep bouts, however this decline was not due to a general decline in signal amplitude over recording time since theta power recovered between REM sleep bouts. Theta power decreased on average by 21% from the 1st 10-second epoch of REM sleep to the midpoint of REM sleep and decreased a further 11% from the midpoint of REM sleep to the last 10-second epoch of REM sleep. This pattern of a significant decrease from the first epoch to subsequent timepoints was evident in, i) short, long, and midlength bouts, ii) single and sequential bouts, iii) interrupted and non-interrupted bouts, and iv) bouts preceding any other state.
Is REM theta power a biomarker of REM sleep homeostatic drive?
Theta may be an indicator of homeostatic drive for REM sleep just as delta power (slow wave activity) is considered an indicator of homeostatic drive for NREM sleep. Delta consistently shows increased power during sleep following prolonged waking and delta power decreases within and between subsequent NREM sleep bouts (Borbely 1982; Tobler and Borbely 1986; Tobler, Franken et al. 1990; Bjorness et al., 2016). Our finding that theta power is higher at the beginning of each REM sleep bout parallels this delta power indicator for NREM sleep. Thus theta power in REM sleep presents what is, to our knowledge, the only homeostatic indicator of REM sleep pressure. Some researchers hypothesize that drive for REM sleep builds during both waking and NREM (Ocampo-Garces, Molina et al. 2000; Franken 2002) while others hypothesize the drive for REM sleep builds exclusively during NREM sleep (Benington, Kodali et al. 1994). We found that the amount of time in NREM sleep between REM sleep bouts was significantly correlated with the theta power decline during REM sleep, with more prior NREM sleep related to larger changes in theta power during REM sleep. This result favors the hypothesis that REM sleep pressure builds exclusively during NREM sleep, not waking. Moreover, we observed that theta decreases just as much across REM sleep bouts in close sequence as those farther apart. Thus, if theta power and declines are a reflection of the homeostatic drive for REM sleep, then even short intervening periods of NREM sleep (i.e. < 3 min) must be enough to re-build homeostatic drive for REM sleep. Further exploration of this hypothesis would analyze theta dynamics within REM bouts across all 24 h of sleep, as there may be circadian effects.
Interestingly, Vyazovskiy and Tobler (2005) have shown that a rise in low frequency theta power during quiet waking predicts the homeostatic increase in slow wave amplitude during subsequent NREM sleep and is thus the best prior indicator of sleep propensity. It would be fascinating to discover which frequency in which state (likely NREM sleep) serves as a prior indicator of REM sleep propensity. Although we saw no change in theta power from the first epoch of the first REM bout to the first epoch of the last REM bout on the second day, and thus no influence of circadian rhythm across the 4 – hour recording period, it would be interesting to look for differences in theta declines between early and late REM bouts in human sleep recordings; both bout length and probability of occurrence of NREM and REM sleep changes with circadian phase across the human sleep period. Our studies suggest that the longer periods of NREM sleep in the early part of the night in humans would lead to larger declines in theta power in those early night REM bouts. However, shorter REM bouts in the early phase of human sleep, combined with our finding of a constant rate of theta power decline no matter what the length of the REM bout, would also conspire against a NREM push toward more theta power decline in early REM sleep periods, necessitating careful study over speculation.
Possible mechanisms underlying theta declines across REM sleep
The processes mediating the decline in theta power across a REM sleep bout could be an important research focus as the same mechanisms could influence the function of REM sleep and influence mental health in disorders involving REM sleep. Some possibilities and their implications are explored here. Decreasing theta power may simply signal the end of a REM sleep bout as its decline is due to increasing desynchronized activity, which is a signature of waking. REM sleep is initiated and controlled by nuclei in the pontine reticular formation (Vertes 1984; Vanni-Mercier, Sakai et al. 1989; Luppi, Gervasoni et al. 2004; McCarley 2004; Siegel 2005). A REM sleep-like state can be induced by injecting cholinergic agonists or GABAergic antagonists into the pontine reticular formation (Baghdoyan, Rodrigo-Angulo et al. 1984; Gnadt and Pegram 1986; Vanni-Mercier, Sakai et al. 1989; Marks and Birabil 1998; Sanford, Tang et al. 2003) while cholinergic cells of the same structure influence both theta generation and its frequency (Bland and Oddie 1998; Bland, Bird et al. 2006; Nunez, de Andres et al. 1991; Vertes, Colom et al. 1993; Kramis and Vanderwolf 1980; Vertes and Kocsis 1997). Stimulating the medial longitudinal fasciculus, the medial forebrain bundle, or the central tegmental tract drives theta (Vertes 1981;Vertes 1982). Conversely, stimulating the serotonergic dorsal raphe nucleus, which is disfacilitated during REM sleep (Vanni-Mercier, Sakai et al. 1989; McGinty and Harper 1976; Trulson and Jacobs 1979), mildly arouses animals (Jacobs, Asher et al. 1973; Yamamoto, Watanabe et al. 1979) and inhibits theta (Yamamoto, Watanabe et al. 1979; Vertes 1981). If the termination of REM sleep is due to a decline in brainstem activity maintaining REM sleep or a rise in wakefulness-promoting activity, these regions controlling both theta and REM sleep may weaken the expression of theta toward the end of a REM sleep bout.
Arguing against a common mechanism of arousal and theta abatement at the end of REM sleep bouts is the fact that REM sleep sometimes directly precedes NREM sleep in which neural activity becomes more synchronized at the transition out of REM sleep rather than more desynchronized. The fall in theta power does not predict which state follows REM sleep. Thus, NREM sleep is as likely as waking to follow a low theta powered REM sleep epoch.
Theta activity is higher during REM sleep phasic activities such as muscle twitches (Whishaw and Vanderwolf 1973; McGinty and Harper 1976; Robinson, Kramis et al. 1977), and rapid eye movements (Sano, Iwahara et al. 1973; Karashima, Nakao et al. 2004) as compared to tonic REM sleep. EMG power during REM sleep is driven by muscle twitches. We found that EMG power was unchanged within REM sleep, increasing only when the animal transitioned to another state, suggesting a lack of change in the rate of phasic muscle twitches across REM sleep. However, a more direct measure of phasic twitches, along with theta frequency and amplitude characteristics, would be beneficial. Interestingly, another indicator of phasic REM sleep, PGO waves, have been shown to decrease in amplitude within REM sleep bouts (Bowker 1985). Decreasing theta power within REM sleep matches well with decreased PGO wave amplitude. Some interventions that increase REM sleep time also increase PGO wave activity (Denlinger, Patarca et al. 1988; Datta, Calvo et al. 1991; Simon-Arceo, Ramirez-Salado et al. 2003). Our results combined with Bowker’s 1985 study would indicate that these increased PGO waves should be clustered toward the beginning of those long REM bouts and/or their amplitudes decrease steadily across the bout.
Cortical temperature increases during the transition from NREM sleep to REM sleep and within REM sleep bouts (Kawamura and Sawyer 1965; Obal, Rubicsek et al. 1985; Wehr 1992; Benington, Kodali et al. 1994). Although theta frequency and cortical temperature are correlated (Deboer 2002; Pan and McNaughton, 1997), theta power has not been studied in relation to brain temperature. Our finding of decreased theta power across REM sleep suggests an inverse relationship between brain temperature and theta power.
Functional consequence of theta declines across REM sleep
Given that theta changes amplitude over a REM sleep bout, it would be interesting to explore whether theta serves a different function at different times of the REM sleep bout. Several theories have been proposed for the function of the theta rhythm, including learning processes (Bunce et al., 2004; Fletcher et al., 2006; Legault, Smith et al., 2004; Leutgeb et al., 1999; Masuoka, Fujii et al. 2006; Mitchell et al., 1982; Winson, 1978) that are mechanistically served by theta-modulated LTP and depotentiation (Pavlides, Greenstein et al. 1988; Huerta and Lisman 1995; Holscher, Anwyl et al. 1997; Hyman, Wyble et al. 2003). Experience-dependent changes in activity are expressed during REM sleep theta patterning (Pavlides and Winson 1989; Poe, Nitz et al. 2000). Finally, hippocampal place cells reactivate during REM sleep at specific phases of the theta rhythm (Poe et al., 2000). A recent study of Boyce et al. (2016) found the suppression of theta generating GABAergic neurons of the basal forebrain exclusively during REM sleep reduced theta power by almost 2/3rds and impaired consolidation of hippocampus dependent tasks. Thus theta power amplitude reduction across a memory consolidation bout could have significant impact on the effectiveness of the memory consolidation process. In light of this consideration, memory reactivation may occur predominantly at the beginning of REM sleep bout when theta power is highest, rather than remaining equally likely throughout. Memory replay could also have different synaptic effects at different theta powers. It would be interesting to test whether the same theta dynamics are present under intensive learning conditions or other non-invasive REM sleep-enhancing interventions such as carbachol (Gnadt and Pegram 1986; Bourgin, Escourrou et al. 1995; Marks and Birabil 1998), vasoactive intestinal peptide (Riou 1982; Bourgin, Lebrand et al. 1997), or changes in ambient temperature (Schmidek, Hoshino et al. 1972; Szymusiak and Satinoff 1981; Rosenthal and Vogel 1993). The sometimes observed spontaneous increases in theta power across a REM sleep bout may also change in frequency under different REM sleep homeostatic drive conditions.
Does abnormal REM sleep have similar theta power declines?
These observed theta power declines within REM sleep bouts may be altered during mental health conditions that have been linked with REM sleep abnormalities. Cortical theta power during REM sleep has been shown to be lower in trauma survivors with post-traumatic stress disorder (PTSD) than in those resilient to PTSD (Cowdin, Kobayashi, and Mellman, 2014). REM sleep has been implicated in resolving emotional memories (Walker and van der Helm, 2009). Perhaps those with low theta power throughout REM sleep are unable to reap the benefit of the processes that can best occur in high theta-powered REM sleep epochs. Further study is also needed to reveal whether theta dynamics during REM sleep affect learning/memory consolidation processes and mental health conditions linked to sleep.
Further investigations should also be conducted to determine whether our observed spontaneous decreases in theta power within REM sleep may be modified by interventions that modify REM sleep homeostatic drive, such as in response to REM sleep deprivation/restriction, or to physical and pharmacologic interventions that influence REM sleep generation and duration, such as ambient temperature and sleeping aids. Although our work supports the idea that REM sleep is homeostatically regulated by the length of the prior NREM period, more work is needed to complete the story of which specific processes of NREM influence REM sleep theta power dynamics and bout lengths.
Conclusion
Our study of brain activity in 20 normally sleeping rats over two days reveals a dynamic diminution of theta within REM sleep cycles that has not previously been described. Such reduction in theta power could both be a biomarker of another underlying process and can itself have important functional consequences for systems regulated by theta. The influence of prior NREM sleep time on theta reduction supports a unique role for theta in recovery from processes occurring during NREM sleep.
Acknowledgements
This research was supported by NIH grant MH60-670 and by the Department of Anesthesiology at the University of Michigan. We thank Apurva Turakhia and Avinash Ananthakrisknan for their assistance with Matlab programming.
References
- Amici R, Zamboni G, et al. (1994). “Pattern of desynchronized sleep during deprivation and recovery induced in the rat by changes in ambient temperature.” J Sleep Res 3(4): 250–256. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, et al. (1984). “Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions.” Brain Res 306(1–2): 39–52. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, et al. (1994). “Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure.” Sleep 17(1): 28–36. [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Riley BT, et al. (2005). “REM restriction persistently alters strategy used to solve a spatial task.” Learn Mem 12(3): 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Dale N, et al. (2016). “An adenosine-mediated glial neuronal circuit for homeostatic sleep.” J Neurosci 36(13): 3906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Bird J, et al. (2006). “Medial septal modulation of the ascending brainstem hippocampal synchronizing pathways in the freely moving rat.” Hippocampus 16(1): 11–19. [DOI] [PubMed] [Google Scholar]
- Bland BH and Oddie SD (1998). “Anatomical, electrophysiological and pharmacological studies of ascending brainstem hippocampal synchronizing pathways.” Neurosci Biobehav Rev 22(2): 259–273. [DOI] [PubMed] [Google Scholar]
- Borbely AA (1982). “A two process model of sleep regulation.” Hum Neurobiol 1(3): 195–204. [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, et al. (1995). “Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat.” Neuroreport 6(3): 532–536. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Lebrand C, et al. (1997). “Vasoactive intestinal polypeptide microinjections into the oral pontine tegmentum enhance rapid eye movement sleep in the rat.” Neuroscience 77(2): 351–360. [DOI] [PubMed] [Google Scholar]
- Bowker RM (1985). “Variability in the characteristics of pontogeniculooccipital spikes during paradoxical sleep.” Exp Neurol 87(2): 212–224. [DOI] [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, Adamantidis AA (2016). “Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation.” Science 352(6287): 812–816. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, et al. (2004). “Timing of administration mediates the memory effects of intraseptal carbachol infusion.” Neuroscience 127(3): 593–600. [DOI] [PubMed] [Google Scholar]
- Cowdin N, Kobayashi I, Mellman TA (2014) “Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD.” Exp Brain Res 232(5): 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Calvo JM, et al. (1991). “Long-term enhancement of REM sleep following cholinergic stimulation.” Neuroreport 2(10): 619–622. [DOI] [PubMed] [Google Scholar]
- Deboer T (2002). “Electroencephalogram theta frequency changes in parallel with euthermic brain temperature.” Brain Res 930(1–2): 212–215. [DOI] [PubMed] [Google Scholar]
- Deboer T and Tobler I, (1995). “Temperature dependence of EEG frequencies during natural hypothermia.” Brain Res 670(1):153–156. [DOI] [PubMed] [Google Scholar]
- Denlinger SL, Patarca R, et al. (1988). “Differential enhancement of rapid eye movement sleep signs in the cat: a comparison of microinjection of the cholinergic agonist carbachol and the beta-adrenergic antagonist propranolol on pontogeniculo-occipital wave clusters.” Brain Res 473(1): 116–126. [DOI] [PubMed] [Google Scholar]
- Fletcher BR, Calhoun ME, et al. (2006). “Fornix lesions decouple the induction of hippocampal arc transcription from behavior but not plasticity.” J Neurosci 26(5): 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, et al. (2007). “Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems.” Behav Brain Res 180(1): 48–61. [DOI] [PubMed] [Google Scholar]
- Franken P (2002). “Long-term vs. short-term processes regulating REM sleep.” J Sleep Res 11(1): 17–28. [DOI] [PubMed] [Google Scholar]
- Gnadt JW and Pegram GV (1986). “Cholinergic brainstem mechanisms of REM sleep in the rat.” Brain Res 384(1): 29–41. [DOI] [PubMed] [Google Scholar]
- Gross BA, Walsh CM, et al. (2009). “Open-source logic-based automated sleep scoring software using electrophysiological recordings in rats.” J Neurosci Meth 184(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Anwyl R, et al. (1997). “Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo.” J Neurosci 17(16): 6470–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT and Lisman JE (1995). “Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro.” Neuron 15(5): 1053–1063. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, et al. (2003). “Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough.” J Neurosci 23(37): 11725–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Asher R, Dement WC (1973). “Electro-physiological and behavioral effects of electrical stimulation of the raphe nuclei in cats.” Physiol Behav 11: 489–495. [DOI] [PubMed] [Google Scholar]
- Karashima A, Nakao M, et al. (2004). “Theta wave amplitude and frequency are differentially correlated with pontine waves and rapid eye movements during REM sleep in rats.” Neurosci Res 50(3): 283–289. [DOI] [PubMed] [Google Scholar]
- Kawamura H and Sawyer CH (1965). “Elevation in brain temperature during paradoxical sleep.” Science 150(698): 912–913. [DOI] [PubMed] [Google Scholar]
- Kramis R and Vanderwolf CH (1980). “Frequency-spe-cific RSA-like hippocampal patterns elicited by septal, hypothalamic, and brain stem electrical stimulation.” Brain Res 192(2): 383–398. [DOI] [PubMed] [Google Scholar]
- Legault G, Smith CT, et al. (2004). “Scopolamine during the paradoxical sleep window impairs radial arm maze learning in rats.” Pharmacol Biochem Behav 79(4): 715–721. [DOI] [PubMed] [Google Scholar]
- Leutgeb S and Mizumori SJ (1999). “Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations.” J Neurosci 19(15): 6661–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, et al. (2004). “Brainstem structures responsible for paradoxical sleep onset and maintenance.” Arch Ital Biol 142(4): 397–411. [PubMed] [Google Scholar]
- Marks GA and Birabil CG (1998). “Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation.” Neuroscience 86(1): 29–37. [DOI] [PubMed] [Google Scholar]
- Masuoka T, Fujii Y, et al. (2006). “Effect of scopolamine on the hippocampal theta rhythm during an eight-arm radial maze task in rats.” Eur J Pharmacol 539(1–2): 76–80. [DOI] [PubMed] [Google Scholar]
- Masuoka T, Fujii Y, et al. (2006). “Participation of the hippocampal theta rhythm in memory formation for an eight-arm radial maze task in rats.” Brain Res 1103(1): 159–163. [DOI] [PubMed] [Google Scholar]
- McCarley RW (2004). “Mechanisms and models of REM sleep control.” Arch Ital Biol 142(4): 429–467. [PubMed] [Google Scholar]
- McGinty DJ and Harper RM (1976). “Dorsal raphe neurons: depression of firing during sleep in cats.” Brain Res 101(3): 569–575. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Rawlins JN, et al. (1982). “Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats.” J Neurosci 2(3): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A, de Andres I, et al. (1991). “Relationships of nucleus reticularis pontis oralis neuronal discharge with sensory and carbachol evoked hippocampal theta rhythm.” Exp Brain Res 87(2): 303–308. [DOI] [PubMed] [Google Scholar]
- Obal F Jr., Rubicsek G, et al. (1985). “Changes in the brain and core temperatures in relation to the various arousal states in rats in the light and dark periods of the day.” Pflugers Arch 404(1): 73–79. [DOI] [PubMed] [Google Scholar]
- Ocampo-Garces A, Molina E, et al. (2000). “Homeostasis of REM sleep after total and selective sleep deprivation in the rat.” J Neurophysiol 84(5): 2699–2702. [DOI] [PubMed] [Google Scholar]
- Olvera-Cortes E, Cervantes M, et al. (2002). “Place-le-arning, but not cue-learning training, modifies the hippocampal theta rhythm in rats.” Brain Res Bull 58(3): 261–270. [DOI] [PubMed] [Google Scholar]
- Pan WX and McNaughton N (1997). “The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity.” Brain Res 764(1–2): 101–108. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, et al. (1988). “Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm.” Brain Res 439(1–2): 383–387. [DOI] [PubMed] [Google Scholar]
- Pavlides C and Winson J (1989). “Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes.” J Neurosci 9(8): 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, et al. (2000). “Experience-depen-dent phase-reversal of hippocampal neuron firing during REM sleep.” Brain Res 855(1): 176–180. [DOI] [PubMed] [Google Scholar]
- Riou F, Cespuglio R, Jouvet M (1982). “Endogenous peptides and sleep in the rat: III The hypogenic properties of vasoactive intestinal polypeptide.” Neuropeptides 2 265–277. [Google Scholar]
- Robinson TE, Kramis RC, et al. (1977). “Two types of cerebral activation during active sleep: relations to behavior.” Brain Res 124(3): 544–549. [DOI] [PubMed] [Google Scholar]
- Rosenthal MS and Vogel GW (1993). “The effect of a 3-day increase of ambient temperature toward the thermoneutral zone on rapid eye movement sleep in the rat.” Sleep 16(8): 702–705. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, et al. (2003). “GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats.” J Neurophysiol 90(2): 938–945. [DOI] [PubMed] [Google Scholar]
- Sano K, Iwahara S, et al. (1973). “Eye movements and hippocampal theta activity in rats.” Electroencephalogr Clin Neurophysiol 35(6): 621–625. [DOI] [PubMed] [Google Scholar]
- Schmidek WR, Hoshino K, et al. (1972). “Influence of environmental temperature on the sleep-wakefulness cycle in the rat.” Physiol Behav 8(2): 363–371. [DOI] [PubMed] [Google Scholar]
- Siegel JM (2005). REM sleep Principles and practice of sleep medicine. Kryger MH, Roth T, Dement WC: 120–135. [Google Scholar]
- Simon-Arceo K, Ramirez-Salado I, et al. (2003). “Long-lasting enhancement of rapid eye movement sleep and pontogeniculooccipital waves by vasoactive intestinal peptide microinjection into the amygdala temporal lobe.” Sleep 26(3): 259–264. [DOI] [PubMed] [Google Scholar]
- Szymusiak R and Satinoff E (1981). “Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate.” Physiol Behav 26(4): 687–690. [DOI] [PubMed] [Google Scholar]
- Tobler I and Borbely AA (1986). “Sleep EEG in the rat as a function of prior waking.” Electroencephalogr Clin Neurophysiol 64(1): 74–76. [DOI] [PubMed] [Google Scholar]
- Tobler I, Franken P, et al. (1990). “Sleep and EEG spectra in the rabbit under baseline conditions and following sleep deprivation.” Physiol Behav 48(1): 121–129. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Tobler I, et al. (1991). “Sleep continuity and the REM-nonREM cycle in the rat under baseline conditions and after sleep deprivation.” Physiol Behav 49(3): 575–580. [DOI] [PubMed] [Google Scholar]
- Trulson ME and Jacobs BL (1979). “Raphe unit activity in freely moving cats: correlation with level of behavioral arousal.” Brain Res 163(1): 135–150. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, et al. (1989). “Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat.” Arch Ital Biol 127(3): 133–164. [PubMed] [Google Scholar]
- Vertes RP (1981). “An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization.” J Neurophysiol 46(5): 1140–1159. [DOI] [PubMed] [Google Scholar]
- Vertes RP (1982). “Brain stem generation of the hippocampal EEG.” Prog Neurobiol 19(3): 159–186. [DOI] [PubMed] [Google Scholar]
- Vertes RP (1984). “Brainstem control of the events of REM sleep.” Prog Neurobiol 22(3): 241–288. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, et al. (1993). “Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat.” Exp Brain Res 96(3): 419–429. [DOI] [PubMed] [Google Scholar]
- Vertes RP and Kocsis B (1997). “Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus.” Neuroscience 81(4): 893–926. [DOI] [PubMed] [Google Scholar]
- Vivaldi EA, Ocampo A, et al. (1994). “Short-term homeostasis of active sleep and the architecture of sleep in the rat.” J Neurophysiol 72(4): 1745–1755. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I (2005). “Theta activity in the waking EEG is a marker of sleep propensity in the rat.” Brain Res 1050: 64–71. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. (2009). “Overnight therapy? The role of sleep in emotional brain processing.” Psychol Bull 135:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb WB and Dreblow LM (1982). “The REM cycle, combining rules, and age.” Sleep 5(4): 372–377. [DOI] [PubMed] [Google Scholar]
- Wehr TA (1992). “A brain-warming function for REM sleep.” Neurosci Biobehav Rev 16(3): 379–397. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Ott T, et al. (1977). “Post-training hippocampal rhythmic slow activity (“theta”) elicited by septal stimulation improves memory consolidation in rats.” Behav Biol 21(1): 32–40. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ and Vanderwolf CH (1973). “Hippocampal EEG and behavior: changes in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats.” Behav Biol 8(4): 461–484. [DOI] [PubMed] [Google Scholar]
- Winson J (1978). “Loss of hippocampal theta rhythm results in spatial memory deficit in the rat.” Science 201(4351): 160–163. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe S, et al. (1979). “Effects of midbrain raphe stimulation and lesion on EEG activity in rats.” Brain Res Bull 4(4): 491–495. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Amici R, et al. (2001). “Pattern of REM sleep occurrence in continuous darkness following the exposure to low ambient temperature in the rat.” Behav Brain Res 122(1): 25–32. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Perez E, et al. (1999). “Control of REM sleep: an aspect of the regulation of physiological homeostasis.” Arch Ital Biol 137(4): 249–262. [PubMed] [Google Scholar]