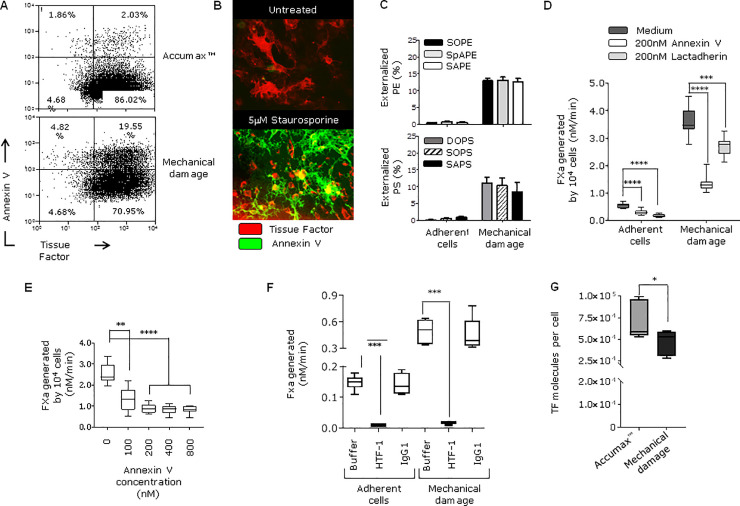
Fig 2. Externalization of PS/PE, expression of TF and pro-coagulant activity on healthy and damaged fibroblasts.
A) Flow cytometry analysis of HCA2 fibroblasts lifted either using Accumax™ cell detachment solution to avoid membrane disruption or by scraping to induce mechanical damage. Cells were stained with Annexin V- Pacific Blue and anti-TF antibody labelled with APC. Plots show cell populations after propidium iodine was used to exclude dead cells. B) Adherent HCA2 fibroblasts were cultured either with medium alone (untreated) or with 5μM staurosporine for 1 hour at 37°C to induce membrane damage due to apoptosis (positive control). Cells were stained with TF antibody (NY2) labelled with APC and Annexin V-A488 for analysis by microscopy. C) Percentage of total and externalized PS and PE species was determined by MS (mean ± SEM) as indicated in materials and methods. Amounts (ng) of externalized PS and PE are presented in Table 1. D) FXa generation was measured on adherent cells and cells after mechanical damage. The effect of PS-binding proteins was assessed by pre-incubating the cells with 200 nM Annexin V or Lactadherin for 30 min at room temperature before adding coagulation factors (FVIIa and FX) ****p <0.0001, ***p <0.001 E) Mechanically-damaged fibroblasts were pre-incubated with increasing concentrations of annexin V prior the addition of FVIIa and FX, ****p <0.0001, **p = 0.005. F) FXa generation by fibroblasts and was measured when cells were adhered or after mechanical damage. TF antibody (HTF-1) or isotype control (10 μg/ml) were pre-incubated with cells before adding FVIIa/FX, and FXa production was assessed, ***p <0.001. G) The number of TF molecules on HCA2 fibroblasts was determined by flow cytometry as described in materials and methods. Cells were labelled with TF antibody-APC after Accumax treatment or scraping. Experiments in this figure were repeated two or three times.