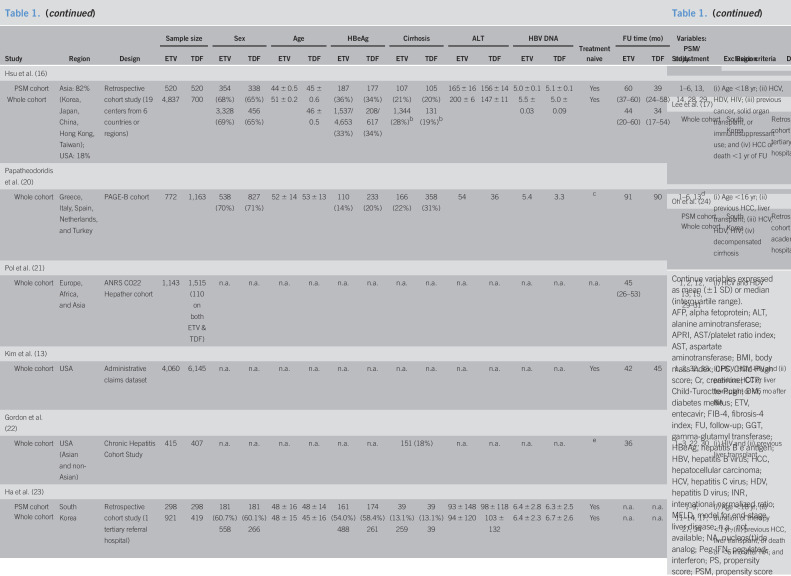
Table 1.
Baseline characteristics of the included studies (n = 13)
| Study | Region | Design | Sample size | Sex | Age | HBeAg | Cirrhosis | ALT | HBV DNA | Treatment naive | FU time (mo) | Variables: PSM/adjustment | Exclusion criteria | ||||||||
| ETV | TDF | ETV | TDF | ETV | TDF | ETV | TDF | ETV | TDF | ETV | TDF | ETV | TDF | ETV | TDF | ||||||
| Kim et al. (15) | |||||||||||||||||||||
| PSM cohort Whole cohort |
South Korea | Retrospective cohort study (1 tertiary referral hospital) | 354 721 |
354 604 |
220 (62%) 471 (65%) |
222 (63%) 363 (60%) |
51 ± 12 52 ± 11 |
52 ± 11 50 ± 11 |
232 (66%) 430 (60%) |
223 (64%) 376 (62%) |
169 (48%) 346 (48%) |
156 (44%) 267 (44%) |
136 ± 159 143 ± 172 |
142 ± 229 137 ± 228 |
6.2 ± 1.4 6.4 ± 1.4 |
6.2 ± 1.5 6.0 ± 1.6 |
Yes Yes |
n.a. 66 (36–88) |
n.a. 33 (21–46) |
1–15 | (i) Decompensated cirrhosis, (ii) FU <1 yr, (iii) Cr >1.5 mg/dL, (iv) HBV DNA <2,000 IU/mL, and (v) death <6 mo or HCC <1 yr after NA |
| Choi et al. (12) | |||||||||||||||||||||
| PSM cohort Whole cohort |
South Korea | Retrospective cohort study (nationwide claims database of NHIS) | 10,923 11,464 |
10,923 12,692 |
6,834 (62%) 11,464 (63%) |
6,834 (63%) 12,692 (63%) |
49 ± 10 49 ± 10 |
49 ± 10 49 ± 10 |
n.a. n.a. |
n.a. n.a. |
2,891 (27%)a 2,991 (26%); D: 450 (4%) |
2,919 (27%)a 3,488 (28%); D: 414 (3%) |
32 (21–54) 32 (21–54) |
35 (24–57) 35 (24–58) |
n.a. n.a. |
n.a. n.a. |
Yes Yes |
51 (38–57) 51 (37–57) |
37 (30–43) 37 (30–44) |
1, 2, 3, 6, 14–20 | (i) Age <30 or >80 yr; (ii) HCV, HDV, HIV; (iii) Previous organ transplant, HCC, or other cancer; and (iv) HCC, transplant, or death <6 mo after NA |
| Kim et al. (18) | |||||||||||||||||||||
| PSM cohort Whole cohort |
South Korea | Retrospective cohort study (4 academic teaching hospitals) | 1,278 1,484 |
1,278 1,413 |
793 (62%) 889 (60%) |
794 (62%) 913 (65%) |
49 ± 11 48 ± 12 |
49 ± 12 49 ± 12 |
758 (50%) 758 (51%) |
727 (50%) 694 (49%) |
476 (32%) 499 (34%) |
456 (32%) 411 (29%) |
n.a. n.a. |
n.a. n.a. |
5.6 ± 2.1 5.7 ± 2.1 |
5.6 ± 2.1 5.4 ± 2.1 |
Yes Yes |
n.a. 59 |
n.a. | 1–4, 8, 9, 13–15 | (i) Age <19 yr; (ii) decompensated cirrhosis; (iii) HCV, HDV; (iv) Previous organ transplant or HCC; (v) HCC, liver transplant, or death <6 mo of enrolment; (vi) and significant medical illness |
| Lee et al. (19) | |||||||||||||||||||||
| PSM cohort Whole cohort |
South Korea | Retrospective cohort study (1 tertiary referral hospital) | 1,370 1,583 |
1,370 1,439 |
806 (59%) 926 (59%) |
798 (58%) 841 (58%) |
47 ± 12 47 ± 12 |
47 ± 11 47 ± 11 |
814 (59%) 974 (62%) |
807 (59%) 823 (57%) |
465 (34%) 567 (36%) |
464 (34%) 483 (35%) |
98 (53–200) 98 (53–201) |
95 (50–196) 94 (51–194) |
6.5 (5–8) 6.5 (5–8) |
6.4 (5–8) 6.4 (5–8) |
Yes Yes |
n.a. 60 |
n.a. 36 |
1–15, 17, 18, 21–25 | (i) HCV and HIV, (ii) HCC and transplant before or <6 mo after NA, (iii) other cancer, and (iv) decompensated cirrhosis |
| Yip et al. (14) | |||||||||||||||||||||
| PSM cohort Whole cohort |
Hong Kong, China | Retrospective cohort study (territory-wide healthcare database of public hospitals) | 4,636 28,041 |
1,200 1,309 |
2,267 (49%) 18,094 (65%) |
587 (49%) 591 (45%) |
43 ± 13 53 ± 13 |
44 ± 13 43 ± 13 |
2,480 (54%) 8,317 (30%) |
625 (52%) 721 (55%) |
167 (4%) 3,822 (14%) |
37 (3%) 38 (3%) |
43 (25–108) 62 (33–137) |
46 (26–107) 43 (25–103) |
4.8 ± 2.8 5.3 ± 2.2 |
4.8 ± 2.7 4.9 ± 2.7 |
Yes Yes |
35 (18–55) 44 (20–60) |
34 (18–54) 34 (17–54) |
1–6, 8–10, 12–15, 26–27 | (i) HCV, HDV, HIV; (ii) autoimmune or metabolic liver disease; (iii) HCC and transplant before or <6 mo after NA; and (iv) FU <6 mo |
| Hsu et al. (16) | |||||||||||||||||||||
| PSM cohort Whole cohort |
Asia: 82% (Korea, Japan, China, Hong Kong, Taiwan); USA: 18% | Retrospective cohort study (19 centers from 6 countries or regions) | 520 4,837 |
520 700 |
354 (68%) 3,328 (69%) |
338 (65%) 456 (65%) |
44 ± 0.5 51 ± 0.2 |
45 ± 0.6 46 ± 0.5 |
187 (36%) 1,537/4,653 (33%) |
177 (34%) 208/617 (34%) |
107 (21%) 1,344 (28%)b |
105 (20%) 131 (19%)b |
165 ± 16 200 ± 6 |
156 ± 14 147 ± 11 |
5.0 ± 0.1 5.5 ± 0.03 |
5.1 ± 0.1 5.0 ± 0.09 |
Yes Yes |
60 (37–60) 44 (20–60) |
39 (24–58) 34 (17–54) |
1–6, 13, 14, 28, 29 | (i) Age <18 yr; (ii) HCV, HDV, HIV; (iii) previous cancer, solid organ transplant, or immunosuppressant use; and (iv) HCC or death <1 yr of FU |
| Papatheodoridis et al. (20) | |||||||||||||||||||||
| Whole cohort | Greece, Italy, Spain, Netherlands, and Turkey | PAGE-B cohort | 772 | 1,163 | 538 (70%) | 827 (71%) | 52 ± 14 | 53 ± 13 | 110 (14%) | 233 (20%) | 166 (22%) | 358 (31%) | 54 | 36 | 5.4 | 3.3 | c | 91 | 90 | 1–6, 13d | (i) Age <16 yr; (ii) previous HCC, liver transplant; (iii) HCV, HDV, HIV; (iv) decompensated cirrhosis |
| Pol et al. (21) | |||||||||||||||||||||
| Whole cohort | Europe, Africa, and Asia | ANRS CO22 Hepather cohort | 1,143 | 1,515 (110 on both ETV & TDF) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 45 (26–53) | 1, 2, 12, 13, 15, 29–31 | (i) HCV and HDV | |
| Kim et al. (13) | |||||||||||||||||||||
| Whole cohort | USA | Administrative claims dataset | 4,060 | 6,145 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Yes | 42 | 45 | 1, 2, 32, 33 | (i) HCV, HDV, HIV and (ii) previous HCC or liver transplant or <6 mo after NA |
| Gordon et al. (22) | |||||||||||||||||||||
| Whole cohort | USA (Asian and non-Asian) | Chronic Hepatitis Cohort Study | 415 | 407 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 151 (18%) | n.a. | n.a. | n.a. | n.a. | e | 36 | 1–3, 22, 30 | (i) HIV and (ii) previous liver transplant | ||
| Ha et al. (23) | |||||||||||||||||||||
| PSM cohort | South Korea | Retrospective cohort study (1 tertiary referral hospital) | 298 921 |
298 419 |
181 (60.7%) 558 |
181 (60.1%) 266 |
48 ± 16 48 ± 15 |
48 ± 14 45 ± 16 |
161 (54.0%) 488 |
174 (58.4%) 261 |
39 (13.1%) 259 |
39 (13.1%) 39 |
93 ± 148 94 ± 120 |
98 ± 118 103 ± 132 |
6.4 ± 2.8 6.4 ± 2.3 |
6.3 ± 2.5 6.7 ± 2.6 |
Yes Yes |
n.a. n.a. |
n.a. n.a. |
1–9, 11–14, 17, 27, 34 | (i) Age <18 yr; (ii) duration of therapy <1 yr; (iii) previous HCC, liver transplant, or death or <6 mo after NA; and (iv) pretreatment HBV DNA <2,000 IU/mL |
| Whole cohort | |||||||||||||||||||||
| Lee et al. (17) | |||||||||||||||||||||
| Whole cohort | South Korea | Retrospective cohort study (1 tertiary referral hospital) | 152 | 49 | n.a. | n.a. | n.a. | n.a. | 0 | 0 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Yes | 60 (range: 24–132) | 1–3, 5–6 | (i) HBeAg positivity, (ii) HCV and HIV, (iii) concomitant chronic liver diseases, (iv) decompensated cirrhosis, and (v) previous HCC | |
| Oh et al. (24) | |||||||||||||||||||||
| PSM cohort | South Korea | Retrospective cohort study (9 academic hospitals) | 516 753 |
516 807 |
319 (61.8%) 480 (63.7%) |
325 (63.0%) 503 (62.3%) |
49 ± 13 49 ± 11 |
49 ± 9 46 ± 11 |
314 (60.9%) 451 (61.4%) |
311 (60.3%) 484 (60.0%) |
238 (46.1%) 315 (41.8%) |
224 (43.4%) 310 (38.4%) |
n.a. n.a. |
n.a. n.a. |
6.4 (5.4–7.5) 6.5 (5.4–7.6) |
6.4 (5.4–7.5) 6.6 (5.5–7.7) |
Yes Yes |
56 (52–64) 59 (53–57) |
58 (47–65) 56.4 (46–65) |
1–5, 8–15, 22, 29, 35, 36 | (i) HCV and HIV, (ii) duration of therapy <1 yr, and (iii) previous HCC or death <1 yr after NA |
| Whole cohort | |||||||||||||||||||||
Continue variables expressed as mean (±1 SD) or median (interquartile range).
AFP, alpha fetoprotein; ALT, alanine aminotransferase; APRI, AST/platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; CPS, Child-Pugh score; Cr, creatinine; CTP, Child-Turoctte-Pugh; DM, diabetes mellitus; ETV, entecavir; FIB-4, fibrosis-4 index; FU, follow-up; GGT, gamma-glutamyl transferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; INR, international normalized ratio; MELD, model for end-stage liver disease; n.a., not available; NA, nucleos(t)ide analog; Peg-IFN, pegylated-interferon; PS, propensity score; PSM, propensity score matched; PT, prothrombin time; RRT, renal replacement therapy; SES, socioeconomic status; TDF, tenofovir.
Decompensated cirrhosis: ETV group 421 (4%), TDF group 388 (4%).
CPS B/C: ETV group 136 (3%), TDF group 29 (4%).
Both treatment naive and experienced.
Not adjusted for HBeAg, previous treatment use, ALT, HBV DNA despite significant difference between ETV and TDF groups.
One hundred sixty-four (20%): only treatment-naive patients were included for analysis in current meta-analysis.
1, age; 2, sex; 3, cirrhosis; 4, HBeAg; 5, HBV DNA; 6, ALT; 7, AST; 8, albumin; 9, bilirubin; 10, creatinine; 11, AFP; 12, INR or PT; 13, platelet; 14, DM; 15, hypertension; 16, smoking; 17, drinking; 18, BMI; 19, SES; 20, healthcare level; 21, APRI; 22, FIB-4; 23, CPS; 24, varix; 25, GGT; 26, RRT; 27, calendar year of treatment initiation; 28, country; 29, hepatic decompensation; 30, ethnicity; 31, fibrosis stage; 32, health conditions; 33, weighting based on treatment PS; 34, HBsAg titer; 35, CTP; 36, MELD.