Table 3.
Summary of adverse events
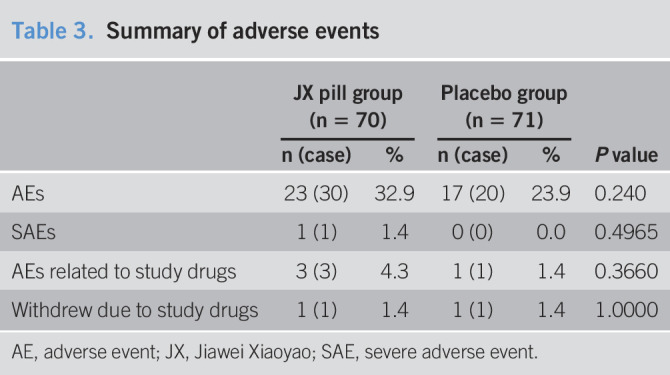
| JX pill group (n = 70) | Placebo group (n = 71) | P value | |||
| n (case) | % | n (case) | % | ||
| AEs | 23 (30) | 32.9 | 17 (20) | 23.9 | 0.240 |
| SAEs | 1 (1) | 1.4 | 0 (0) | 0.0 | 0.4965 |
| AEs related to study drugs | 3 (3) | 4.3 | 1 (1) | 1.4 | 0.3660 |
| Withdrew due to study drugs | 1 (1) | 1.4 | 1 (1) | 1.4 | 1.0000 |
AE, adverse event; JX, Jiawei Xiaoyao; SAE, severe adverse event.
