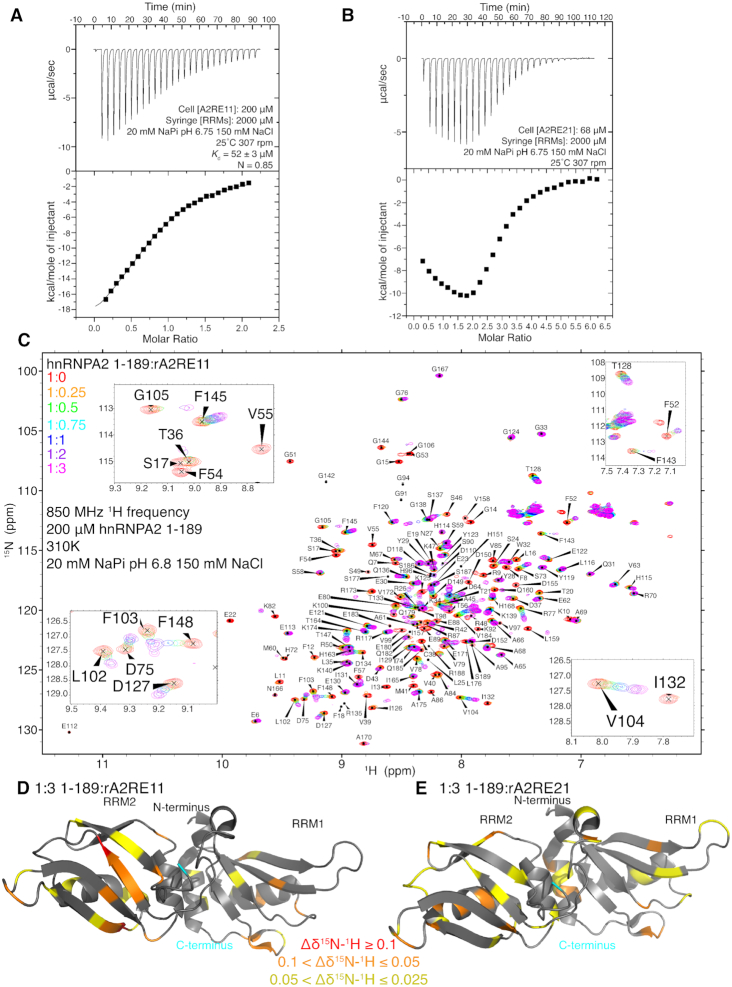
Figure 2.
hnRNPA2 1–189 weakly binds the A2RE RNA. See also Supplementary Figure S2. (A) ITC of the hnRNPA2 1–189 binding to rA2RE11. Dissociation constant is about 52 μM and the interaction stoichiometry is closest to 1 to 1. (B) ITC of hnRNPA2 1–189 binding to rA2RE21. The isotherm has a distinctive biphasic curve and inflection point at or above 2.0, consistent with multiple binding site scheme (i.e. at least, and most likely, two hnRNPA2 1–189 can simultaneously bind rA2RE21). (C) 1H–15N HSQC of hnRNPA2 1–189 titrated with increasing concentrations of rA2RE11. Data is consistent with weak binding with most peaks being in intermediate-fast exchange. Insets show a number of peaks that shift (e.g. T128, F145, V104), decrease signal intensity (e.g. I132, F54), or shift and decrease then increase signal intensity (e.g. G105, F143). (D) Average chemical shift deviations for the titration of rA2RE11 (from S3A-B) plotted on PDB structure of hnRNPA2 RRMs 5HO4. (E) Average chemical shift deviations for the titration of rA2RE21 (from S3D–E) plotted on 5HO4.