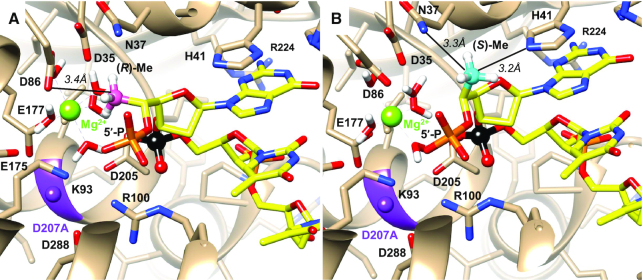
Figure 10.
Crystal structure of the D. melanogaster Xrn1 5′ exonuclease active site bound to 5′-P-d(TTT)-3′ in which the 5′-terminal base was replaced with (A) 5′-(R)-C-methyl-guanine (pink) and (B) 5′-(S)-C-methyl-guanine (cyan). Models were energy minimized. Selected side chains are labeled, a bound Mg2+ is shown as a green sphere, the position of D207, which was mutated to alanine to inactivate the nuclease by preventing binding of the second metal ion to facilitate crystallization, is indicated in purple, and the scissile phosphate is highlighted in black. The shortest distances between methyl carbons and side chain atoms are shown as thin solid lines.