Figure 4.
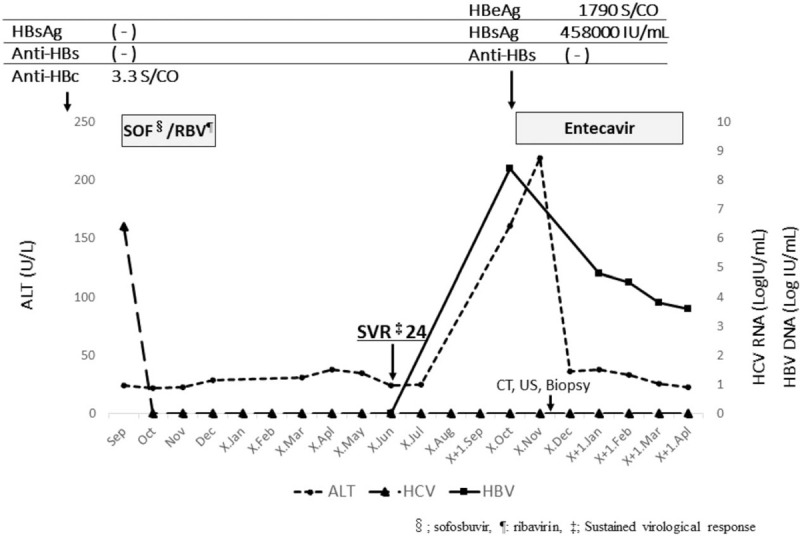
Clinical course. Hepatitis B surface antigen (HBsAg) and antibody against HBsAg were negative, antibody against hepatitis B virus (HBV) core was positive before direct-acting antiviral treatment. Treatment with sofosbuvir (SOF) + ribavirin (RBV) promptly decreased serum hepatitis C virus RNA levels, thereafter hepatitis C virus RNA remained undetectable. Serum alanine transaminase suddenly increased after treatment with SOF + RBV. Simultaneously, HBsAg became positive, while anti-HBs remained negative, and serum HBV DNA level increased, reaching 8.9 LogIU/mL at 39 weeks post cessation of direct-acting antiviral treatment with SOF + RBV. Entecavir was then administered. Liver function improved upon initiation of entecavir treatment. Moreover, serum HBV-DNA level gradually decreased.
