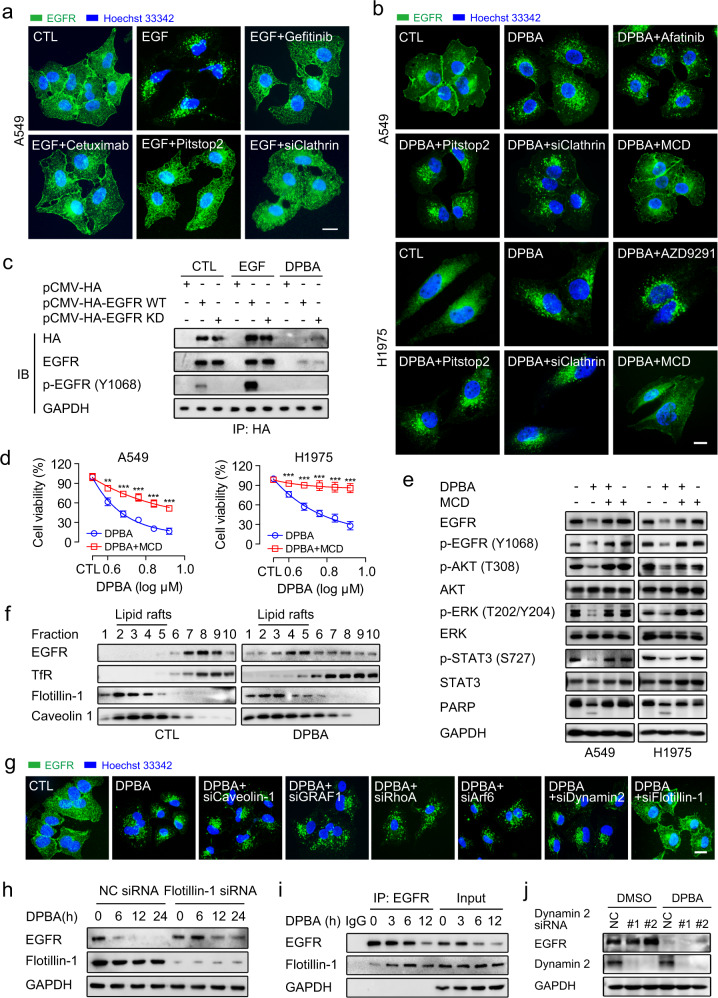
Fig. 3.
DPBA-induced EGFR endocytosis is clathrin-independent but lipid raft-mediated. a EGF-induced EGFR endocytosis was impaired by inhibition of EGFR kinase activity or clathrin. A549 was treated with EGF (20 ng/ml) in the presence or absence of gefitinib (10 μM), cetuximab (5 μg/ml), pitstop2 (5 μM), or clathrin siRNA for 30 min. EGFR endocytosis was observed by immunofluorescence (magnification, ×630; scale bar, 10 μm). b DPBA-induced EGFR endocytosis was blocked by a lipid raft inhibitor MCD. A549 and H1975 were treated with DPBA (6 μM) in the presence or absence of afatinib (10 μM), AZD9291 (10 μM), pitstop2 (5 μM), MCD (1 mg/ml), or clathrin siRNA for 6 h. EGFR endocytosis was observed by immunofluorescence (magnification, ×630; scale bar, 10 μm). c DPBA degraded both EGFR WT and EGFR KD. HEK-293T transfected with pCMV-HA, pCMV-HA EGFR WT, or pCMV-HA EGFR KD was treated with EGF (20 ng/ml) for 30 min or DPBA (6 μM) for 24 h. Exogenous EGFR was subjected to pull-down with anti-HA antibody. EGFR and p-EGFR (Y1068) levels were measured by Western blot. d A549 and H1975 were treated with indicated concentrations of DPBA in the presence or absence of MCD (1 mg/ml) for 24 h. Cell viability was measured by MTT assay, **P < 0.01, ***P < 0.001 vs. DPBA, n = 3. e A549 and H1975 were treated with DPBA (6 μM) with or without MCD (1 mg/ml) for 24 h. Total EGFR, p-EGFR (Y1068), Akt, p-Akt (T308), ERK, p-ERK (T202/Y204), STAT3, p-STAT3 (S727), and PARP expression levels were measured by Western blot. f DPBA induced EGFR accumulation in lipid rafts. A549 was treated with DPBA (6 μM) for 3 h. EGFR distributions in lipid rafts were detected by density gradient centrifugation and Western blot. TfR was a non-lipid raft marker while caveolin-1 and flotillin-1 were lipid raft markers. g Flotillin-1 knockdown blocked DPBA-induced EGFR endocytosis. A549 was transfected with dynamin 2 siRNA, flotillin-1 siRNA, caveolin-1 siRNA, GRAF1 siRNA, RhoA siRNA, and Arf6 siRNA for 48 h, followed by DPBA treatment (6 μM) for 6 h. EGFR endocytosis was observed by immunofluorescence (magnification, ×630; scale bar, 10 μm). h Flotillin-1 knockdown inhibited DPBA-induced EGFR degradation. A549 was transfected with flotillin-1 siRNA (100 nM) for 48 h, followed by DPBA treatment (6 μM) for 24 h. EGFR degradation was detected by Western blot. i DPBA enhanced interaction of EGFR and flotillin-1. A549 was treated with DPBA (6 μM) for 3, 6, and 12 h. Interactions between EGFR and flotillin-1 were detected by co-IP. j A549 was transfected with dynamin 2 siRNA (100 nM) for 48 h, followed by DPBA treatment (6 μM) for 24 h. EGFR degradation was detected by Western blot