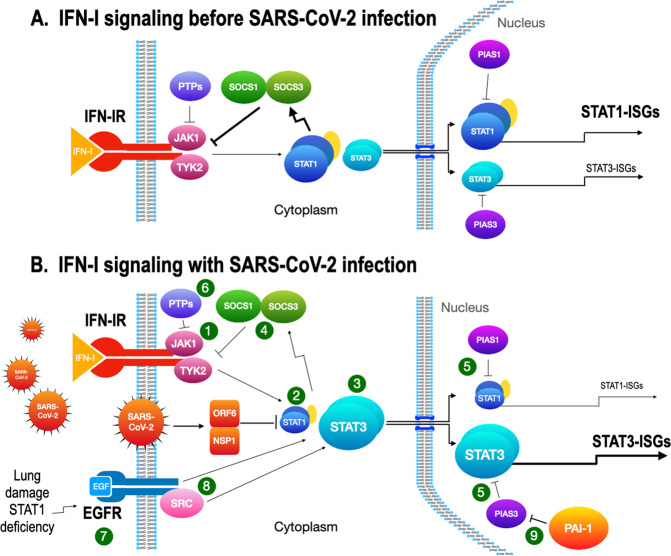
Fig. 3. STAT1- and STAT3-dependent drug targets in IFN-I signaling.
A IFN-I signaling before SARS-CoV-2 infection. JAK1 and TYK2 (1) are activated after IFN-I stimulation. STAT1 is normally activated in IFN-I signaling to induce ISGs (STAT1-ISGs) by ISGF3 (STAT1/STAT2/IRF9). STAT3 is also activated and becomes a homodimer, but the response is small. B IFN-I signaling with SARS-CoV-2 infection. After the infection, STAT1 activity is inhibited by the SARS-CoV-2 proteins, NSP1, and ORF6 (2). With STAT1 activity restricted, STAT3 (3) then becomes dominant and induces STAT3-ISGs. Both STAT1 and STAT3 induce SOCS1 and SOCS3 (4) that inhibit the kinase activity of JAKs for the negative feedback of IFN-I signaling. PIAS1 and PIAS3 (5) inhibit the binding of STAT1 and STAT3 to DNA, respectively, to regulate IFN-I signaling. The role of PIAS3 becomes critical when STAT3 is aberrantly activated and uncoupled from SOCSs regulation. Protein tyrosine phosphatases (PTPs, 6) have regulatory activities on activated JAKs and STATs, but their role in the viral infection needs further clarification. EGFR (7) is upregulated by acute lung injury or by reduced STAT1 activity in the SARS-CoV-2-infected lung. STAT3 is activated through directly binding to EGFR, through EGFR-activated SRC (8), or through JAK2 (data not shown). PIAS3 normally limits the activity of STAT3 but PAI-1 produced during infection blocks PIAS3 activity (9) and an escalating cascade in the STAT3/PAI-1 axis is established.