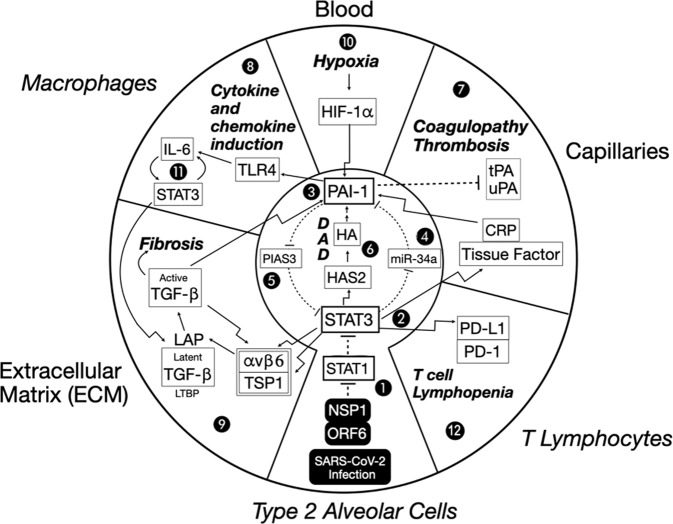
Fig. 4. A dysregulated STAT3-PAI-1 signaling node is common to COVID-19 pathophysiology. Proposed role of STAT3-PAI-1 signaling node in catastrophic cascades underlying COVID-19 pathophysiology.
Infection by SARS-CoV-2 intracellularly delivers NSP1 and ORF6, which efficiently inhibit STAT1 function (1). Repression of STAT1 increases STAT3 (2) activity. STAT3 upregulates PAI-1 (3) by repressing miR-34a, a PAI-1 inhibitor (4). This increased PAI-1 reciprocally activates STAT3 by blocking PIAS3, a STAT3 inhibitor (5). STAT3 can activate HAS2, a hyaluronic acid synthase, which produces hyaluronan (HA) and leads to diffuse alveolar damage (DAD) characterized by hyaline membrane formation (6). Fragments of HA (LMW-HA) activate PAI-1 (3). An escalating cycle of stimulation between STAT3 and PAI-1 begins a catastrophic cascade of events, resulting in combinations of coagulopathy/thrombosis (7), macrophage production of cytokines and chemokines (8), and profibrotic changes (9). Hypoxia eventually results, which further induces PAI-1 transcription through HIF-1α (10). This elevated PAI-1 activity then drives IL-6 production via TLR4, which in turn stimulates even more STAT3 activity (No. 11). Elevated STAT3 also activates PD-L1 in endothelial cells, leading to T cell lymphopenia (No. 12). Details of these events are described in the main text. Italicized outside labels are cell types, and non-italicized outside labels are locations. Bolded italicized text indicates disease states. Bent arrows indicate the transcriptional induction of the indicated target proteins. Straight arrows indicate direct activation. Dashed lines indicate direct inhibition.