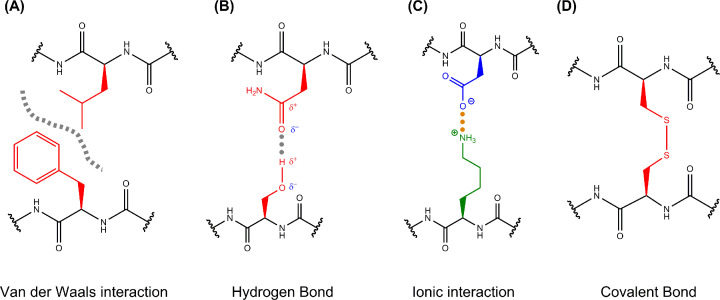
Figure 2. Intermolecular interactions.
Interactions between amino acid side chains help to stabilise the folded structures of proteins and allow proteins to interact with each other. These interactions can include (A) van der Waals interactions when molecules with complementary shapes approach each other. These molecules can be uncharged and only contain non-polar bonds yet at close contact, an instantaneous dipole can be induced in these non-polar bonds allowing weak electrostatic interactions between oppositely (partially) charged groups. Although an individual van der Waals force is weak, many such interactions across non-polar surfaces can allow two proteins to interact with each other. Non-polar groups can also be attracted to each other through the hydrophobic effect, which will be considered when discussing protein folding. (B) Hydrogen bonding occurs when two interacting molecules each contain dipoles (i.e. they contain polar covalent bonds), where the electrostatic attraction occurs between a partially negative N or O atom (with a lone pair of electrons) and a partially positive hydrogen atom that is covalently bound to a different N or O atom. Unlike van der Waals interactions, these bonds are not just dependent on the magnitude of the partial charges and the distance between them but also dependent on orientation of the groups involved. When the Hydrogen is linear with the covalently attached N or O and the interacting N or O (i.e. all three atoms and the lone pair of electrons appear on a line) the strength is maximal. As such, the proteins must fold and interact with other proteins using very precise geometries that satisfy this directional dependence in order to form hydrogen bonds that are strong and significant. (C) Ionic interactions (salt bridges) are attractive interactions between oppositely charged ions, since ions contain more charge than the other dipoles discussed above, they are the strongest intermolecular interaction involving charge, and (D) disulphide bonds are sulphur–sulphur covalent bonds formed by the oxidation of two cysteine residues which can be formed within a single protein chain or between two separate chains. Given these bonds are covalent, they are the strongest overall intermolecular bond, however, the bonds can be broken if a protein is exposed to reducing environments and becomes reduced.