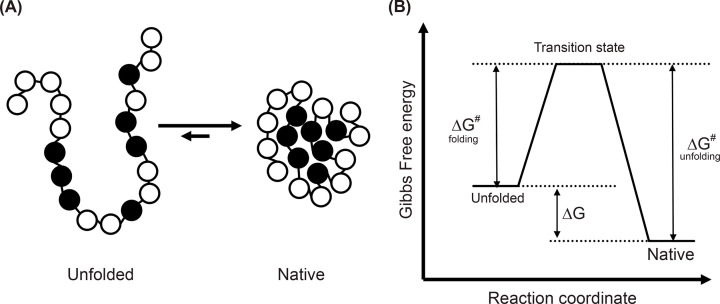
Figure 7. Two state folding of a small protein.
(A) Hydrophobic collapse. In the compact fold (to the right), the hydrophobic amino acids (shown as black spheres) collapse towards the centre to become shielded from aqueous environment. (B) The classical view of protein folding. Diagram represents the free energy of the native and denatured ensembles of a protein under conditions where the native state is favoured as the native state has a lower free energy than the unfolded state. The free energy difference between these states (ΔG) is a measure of the stability of the protein. The transition state ensemble is a population of short-lived and partially folded conformations that cannot be directly observed in experiments but must be passed through to fold and defines the activation barrier for folding (ΔG# folding) and unfolding (ΔG# unfolding).