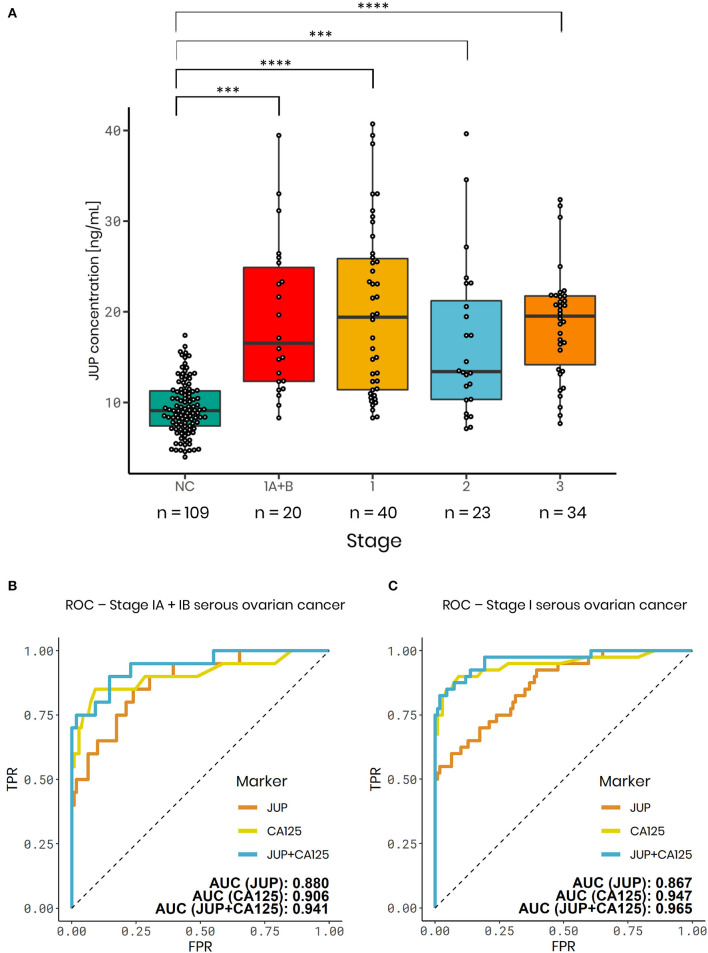
Figure 3.
JUP plasma ELISA results for the international, multicenter serous ovarian cancer validation cohort. (A) Boxplots of normal controls (NC) (median = 9.09 ng/mL), serous ovarian carcinomas - Stage IA+B (median = 15.45 ng/mL), stage I (median = 18.13 ng/mL), stage II (median = 13.49 ng/mL), and stage III (median = 19.52 ng/mL). Serous carcinomas vs. NC (n = 109) - Stage IA+B (1.97-fold change; p < 0.001; n = 20); stage I (2.09-fold change; p < 0.0001; n = 40); stage II (1.81-fold change; p < 0.001; n = 23) and stage III (1.98-fold change; p < 0.0001; n = 34). (B,C) ROC analysis of JUP (orange), CA125 (yellow) and a logistic regression model combining JUP + CA125 (blue). AUC values: (B) Serous carcinomas - Stage IA+B: JUP: 0.880; CA125: 0.906; JUP + CA125: 0.941. (C) Serous carcinomas - Stage I: JUP: 0.867; CA125: 0.947; JUP + CA125: 0.965. ***p-value < 0.001, ****p-value < 0.0001.