Abstract
The hepatitis E virus (HEV) is the fifth known form of viral hepatitis and was first recognized as the cause of an epidemic of unexplained acute hepatitis in the early 1980s. Globally, it is one of the most frequent causes of acute viral hepatitis. The majority of HEV infections are asymptomatic and lead to the spontaneous clearance of the virus. Among the eight different genotypes identified to date, HEV genotype 1 (HEV1), HEV2, HEV3, and HEV4 are the most frequent genotypes causing infections in humans. HEV1 and HEV2 are prevalent in developing regions and able to result in large-scale outbreaks originating from contaminated water supplies. They are also responsible for severe hepatitis in pregnant patients and infants. In contrast, HEV3 and HEV4 are zoonotic, and the transmission of these genotypes to humans occurs mainly through the fecal contamination of water and consumption of contaminated meat from infected animals. Their main reservoir is the pig, and they are mostly encountered in developed countries. The major risk groups for HEV infection and its ensuing adverse consequences are pregnant women, infants, older people, immunocompromised individuals, patients with underlying chronic liver diseases, and workers that come into close contact with HEV-infected animals. In the clinical perspective, HEV infections have diverse clinical manifestations including acute and self-limiting hepatitis, acute-on-chronic liver disease, chronic hepatitis, cirrhosis, and liver failure. Although HEV mainly results in acute self-limiting infection, chronic HEV infection may occur among immunocompromised patients (e.g., solid-organ transplant recipients). Additionally, HEV-associated extrahepatic manifestations involving various organs have been reported in the last decade, although the causal link for many of them still needs to be proven. Ribavirin and interferon-alpha are the most widely used agents for the treatment of HEV infections with a certain level of success. However, ribavirin is contraindicated in pregnant patients, and interferon-alpha cannot be used in most transplant recipients. Therefore, there is an urgent need for novel antiviral compounds that are safe and effective particularly for patients having contraindications for ribavirin or interferon-alpha and infected by the ribavirin-resistant HEV. In this review article, a literature search using PubMed and MEDLINE databases was performed, up to March 2020. Only the articles published in English were reviewed.
Keywords: Hepatitis E, Hepatitis E virus, Extrahepatic manifestations, Zoonotic infection, Chronic hepatitis, Acute hepatitis
Core Tip: The hepatitis E virus (HEV) is the most common cause of acute viral hepatitis worldwide. To date, four main genotypes of the HEV infecting humans have been described. While HEV1 and HEV2 cause only acute hepatitis, HEV3 or HEV4 can become chronic in immunocompromised patients. Extrahepatic manifestations have also been defined for these genotypes. Acute infections are generally self-limiting and do not require special treatment. For chronic hepatitis, ribavirin is the drug of choice. Nevertheless, novel drugs are required for patients in whom ribavirin treatment fails. We herein reviewed the epidemiology, diagnosis, clinical manifestations, and treatment of HEV infections.
INTRODUCTION
The hepatitis E virus (HEV) is the most common cause of acute viral hepatitis worldwide and belongs to the Hepeviridae family[1-3]. Despite being an important cause of hepatitis and increasing knowledge on the HEV, the origin of the HEV remains obscure[4]. In 1983, the Russian virologists Balayan et al[5] visualized the virus by electron microscopy while examining one of their own feces after ingestion of a pooled fecal extract of infected soldiers.
The HEV is a small non-enveloped virus, 27-34 nm in diameter, with a single-stranded positive sense ribonucleic acid (RNA) genome[6,7]. The HEV genome harbors discontinuous regions called open reading frames (ORF). Among these regions, ORF1 encodes nonstructural (functional) proteins (e.g., RNA-dependent RNA polymerase, methyltransferase)[8,9], ORF2 encodes the viral capsid protein[10], and ORF3 encodes a functional ion channel that has important roles in the release of viral particles[11]. The recently discovered ORF4 is unique for HEV genotype 1 (HEV1) and plays a critical role in the proper functioning of HEV RNA polymerase[12]. The capsid protein encoded by ORF2 is highly immunogenic, and antibodies against this protein have neutralizing and protective features[13,14]. Therefore, the capsid protein seems to be a suitable target for vaccine development against HEV. HEV molecular biology is outside the scope of this review. Therefore, we direct the readers to other review articles on this topic[15,16].
Our viewpoint of the HEV has undergone a dramatic change over the past decade. Previously, HEV was considered to be limited to some developing countries. Currently, it is known to be endemic as a zoonotic infectious agent in most high-income countries. Locally acquired (autochthonous) HEV infections caused by HEV3 and HEV4 have become the most common cause of acute viral hepatitis in several developed countries. The best example of this is China where previously HEV1 was the most frequent genotype. However, HEV4 has surpassed HEV1 in recent years, most probably due to improved sanitation and hygiene measures[17].
In this review, we aimed to present contemporary data about the epidemiology, diagnosis, clinical manifestations, and management of HEV infections.
EPIDEMIOLOGY
Among the eight distinct HEV genotypes that have been identified in the Orthohepevirus A species, HEV1, HEV2, HEV3, and HEV4 are able to infect humans. Humans are the main reservoir of HEV1 and HEV2, and any transmission from animals to humans for HEV1 and HEV2 has not yet been reported. The epidemics of HEV1 and HEV2 develop periodically in several regions of Asia, Africa, Mexico, and the Middle East[18]. In these regions, large waterborne outbreaks can be caused by the inadvertent fecal contamination of water supplies particularly after heavy rainfall and flooding[19,20]. In 1955-1956, the first identified HEV outbreak had infected 29300 individuals in India[19]. In addition to the epidemic infection, sporadic HEV infections have occurred in endemic areas[21].
Although HEV1 and HEV2 usually lead to self-limiting acute viral hepatitis, HEV1- and HEV2-related infections still have a substantial burden on public health in low-income countries. According to the mathematical model developed in 2005, these genotypes were associated with 20.1 million annual new infections in Asia and Africa with 3.4 million symptomatic hepatitis E cases, 70000 fatalities attributed to acute liver failure, and 3000 stillbirths[22]. However, these estimates have several restrictions and require updating. The person-to-person transmission of HEV1 and HEV2 is infrequent in both sporadic and epidemic settings[23], whereas vertical transmission from mother to fetus during pregnancy is well defined[24]. Moreover, HEV1 transmission through blood transfusion was also reported[25]. For unknown mechanisms, the mortality rate of acute HEV1 or HEV2 infection is considerably high in pregnant women and infants.
HEV3 and HEV4 infections mainly develop through zoonotic transmission that is caused by close contact with infected animals or the consumption of contaminated food products (most commonly raw or undercooked meat). The main reservoir for these genotypes is the pig, and they have also been demonstrated in wild boars, rabbits, goats, sheep, deer, horses, cats, and dogs[26-29]. Therefore, these animals can be considered as potential zoonotic sources for transmission to humans. They can also be detected in large quantities in the feces of asymptomatic animals and in the milk of infected cows[30]. Some experiments revealed that the HEV can be inactivated if heated up to 71 °C for at least 20 min[31]. In addition, fruits and vegetables washed with contaminated waters can be putative routes of HEV transmission[32,33]. Considering the transmission routes of HEV3 and HEV4, we can conclude that farmers, veterinarians, and individuals working in the slaughterhouse are more prone to HEV infections than the general population.
Albeit less common than zoonotic and waterborne transmissions, infected blood or blood products from a viremic patient should be considered as a possible transmission route for HEV infections[34]. However, most of iatrogenic transmissions remain asymptomatic in immunocompetent individuals. A study from the United Kingdom involving 225000 blood donations demonstrated that 0.035% of recipients were viremic, and almost 42% of recipients being transfused with HEV RNA-positive blood products became viremic or developed antibodies against HEV[35]. Currently, blood products are routinely tested for HEV RNA in the United Kingdom, Ireland, and the Netherlands[36]. Selective screening is performed in Germany and France for high-risk patients, and authorities in Greece, Portugal, Italy, and Spain are assessing whether to initiate HEV screening in blood products[36]. A study from the United Kingdom showed the cost-effectiveness of routine screening of the HEV in solid-organ transplant recipients by the nucleic acid amplification tests (NAATs) or antigen test[37]. The screening of plasma-derived blood products in the United States may not be necessary since only 0.002% of plasma donations in the United States were found HEV RNA positive[38]. In addition to blood products, HEV acquisition can occur from a transplanted organ. Schlosser et al[39] reported a case of a liver transplant recipient who experienced chronic HEV infection and cirrhosis after receiving an organ from a donor with negative HEV serology.
In developed countries, HEV3 is the most frequent causative genotype in HEV infections[21]. However, HEV4 has become as equally important as HEV3 in China, Japan, Taiwan, Hong Kong, and South Korea over the last decades[40]. In Europe, the prevalence of HEV infections varies according to regions. In particular, HEV3 is hyperendemic in southwest France with a very high rate of seroprevalence (> 50%)[41]. It is also endemic in northern France, Belgium, the Netherlands, and Germany. The rates of previous HEV exposure can reach up to 30% in these countries[21]. In a survey conducted in 30 European countries, the number of cases with HEV infection has increased from 514 per year in 2005 to 5617 in 2015[42]. In addition, HEV infection was reported as the most frequent type of acute viral hepatitis between 2013 and 2015 in the Netherlands[43]. These infections are generally locally acquired (autochthonous), and both symptomatic infections and seroprevalence rates increase significantly with age. In the United States, a recent study reported an HEV seroprevalence rate of 6%[44]. The lower rate of seropositivity in the United States compared with that in other developed countries, especially in Europe, can be partly explained by less frequent organ meat consumption, insufficient awareness of HEV among United States health-care providers, and lack of a Food and Drug Administration-licensed assay to diagnose HEV infection[45]. Turkey has been reported as an HEV1 and HEV2 endemic country based upon outdated data. However, for the first time in the literature, we have identified HEV3 viremia in two Turkish patients involved in a cross-sectional study including the high-risk groups for HEV infection, and there was no HEV1 or HEV2 viremic patient (unpublished data).
Thus far, HEV5 and HEV6 have only been reported in wild boars and are not associated with infections in human beings[46]. HEV7 and HEV8 have been detected in camels[47]. In 2016, HEV7 was detected for the first time in a patient regularly consuming camel milk and meat from the United Arab Emirates[48]. Phylogenetic analysis proved that the viruses identified in the samples obtained from the patient and samples from camel meat and milk belong to HEV7. However, no further cases in humans have been identified since then. In addition to these well-defined HEV genotypes infecting humans, some recent studies found that a new HEV genotype called HEVC1 that is normally infecting rats leads to acute and/or chronic hepatitis as well as extrahepatic manifestations in humans[49].
The estimation of HEV seroprevalence by examining the frequencies of anti-HEV antibodies (immunoglobulin (Ig)G and IgM) in a population would be extremely difficult considering the use of different assays with varied sensitivities in the detection of anti-HEV antibodies. For instance, the seroprevalence rate of anti-HEV antibodies in Asia and Africa was 10%-40% with increasing frequency in older age groups (> 50 years of age)[50]. This low rate of anti-HEV antibody positivity in these endemic regions could be explained by the disappearance of anti-HEV antibodies with time, failure of the surveillance systems to detect symptomatic HEV cases, or low sensitivity of previously used serological assays[45]. Nevertheless, new-generation serological assays are more sensitive and have similar specificity than older widely used serological assays[51,52].
DIAGNOSIS
The incubation period of HEV infection is usually 2-6 wk[51]. At the time of diagnosis, HEV RNA and anti-HEV IgM can be detected, followed by anti-HEV IgG antibodies. Anti-HEV IgM antibody positivity in the serum can be considered an important marker for acute HEV infection. Anti-HEV IgM antibodies have a positivity for a short period of time (approximately 3-4 mo), but sometimes it persists for a year[53]. Anti-HEV IgG antibody is relatively long-lasting, and the exact duration of this response remains uncertain[45]. Furthermore, anti-HEV IgG antibody has an increasing avidity over time[54]. HEV RNA can be detected in the blood after 3 wk of exposure, and viral shedding lasts approximately 4-6 wk in the stool[54].
Enzyme immunoassay is the most widely used serological method for the identification of anti-HEV IgG and IgM antibodies in the diagnosis of HEV infection. The detection of anti-HEV IgM and rising titers of anti-HEV IgG antibodies alone are not sufficient for diagnosis since some commercial assays have insufficient specificities for these antibodies. In addition, anti-HEV IgM may disappear or not yet become positive until a sample is taken for the diagnosis of HEV infection. Moreover, anti-HEV IgG antibodies do not provide lifelong immunity, and the level of antibodies in the serum decreases over time. It has been suggested that having anti-HEV IgG titers < 7 units/mL is insufficient to prevent subsequent acute or chronic infections[55]. Conversely, a vaccine study suggested that anti-HEV IgG titers > 2.5 units/mL are protective[23].
Enzyme immunoassays are used not only for the detection of anti-HEV antibodies but also for that of the HEV capsid antigen. Although previously used antigen assays are not as sensitive as the NAATs, a newer version of assays has improved sensitivity[56,57]. Strikingly, HEV antigen may persist for several months after ribavirin-induced HEV RNA clearance of chronic HEV infection. This finding suggests that the presence of HEV antigen does not indicate the presence of infectious virions[58]. Therefore, the role of HEV antigen in diagnosis remains to be determined.
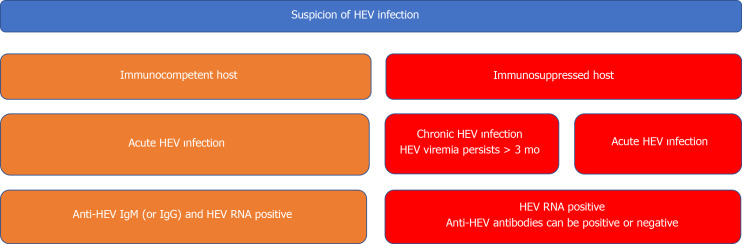
The detection of HEV RNA in the blood or stool is a diagnostic means for HEV infection. Particularly, the results of serological assays are often negative in acute and chronic HEV infections of patients with immunosuppressed conditions (e.g., solid-organ transplant recipients). Chronic HEV infection is defined as the persistence of HEV RNA in the blood or stool for at least 3 mo[59]. Different types of the NAATs have various sensitivities in the detection of HEV RNA. Therefore, the World Health Organization developed the international standard and international reference panel for HEV1, HEV2, HEV3, and HEV4[60]. This enabled us not only to compare the results obtained by different NAATs but also to report the results using a common unit, that is, the international unit (IU). Several different types of the NAATs are being used for the detection of HEV RNA in blood or stool samples. These tests can be exemplified as follows: Conventional reverse transcription polymerase chain reaction (RT-PCR), real-time RT-PCR, and reverse transcription loop-mediated isothermal amplification. The NAATs are able to detect the HEV RNA target, specifically conserved domains of the HEV genome (the region of ORF2 that overlaps ORF3), of all four major genotypes of HEV (genotypes 1-4) that infect humans[61]. However, polymorphisms in the targeted regions or problems in the designation of highly specific primers and probes' sequences may result in false-negative test results. The diagnostic algorithm of both acute and chronic HEV infections is shown in Figure 1.
Figure 1.
Diagnostic algorithm of hepatitis E virus infection. For diagnosis of acute hepatitis E virus (HEV) infection in immunocompetent patients, both serologic assays and nucleic acid amplification tests (PCR) should be used in combination. Negative PCR results can be seen in early period of acute infection. On the other hand, serologic tests are not reliable tools in immunosuppressed patients. Therefore, PCR results are much more important in diagnosis of acute infection among these patients. In addition, HEV RNA positivity that is lasting at least 3 mo is accepted as a diagnostic marker for chronic HEV infection in some immunocompromised patients (e.g., solid-organ transplant recipients, allogeneic hematopoietic stem cell transplant recipients). HEV: Hepatitis E virus; RNA: Ribonucleic acid; Ig: Immunoglobulin.
CLINICAL MANIFESTATIONS OF HEV INFECTIONS
Acute HEV infections
In general, acute HEV infection is relatively asymptomatic or mildly symptomatic. However, acute icteric hepatitis is seen in almost 5%-30% of patients infected by the HEV[62]. Malaise, fever, body aches, nausea, and vomiting are characteristic symptoms observed through the 1-wk prodromal phase of acute icteric hepatitis, which is followed by the icteric phase lasting approximately 1 wk. It is marked by dark-colored urine and jaundice[62]. Then, the convalescent phase results in the resolution of icteric symptoms. Generally, HEV1 and HEV2 cause more severe acute hepatitis presentation than HEV3 and HEV4[63]. Nonetheless, HEV3 and HEV4 may lead to severe acute HEV infections in older men and acute-on-chronic liver failure (ACLF) in patients with chronic liver diseases.
Although the vast majority of acute HEV infections do not need any special treatment in immunocompetent patients, it is consistently shown that HEV1 infection in a pregnant woman (particularly at the third trimester) is associated with high maternal morbidity and mortality. Acute HEV1 infection-related mortality reaches up to 20% and is caused by eclampsia, hemorrhagic complications, and liver failure[64]. The newborns have a risk of maternal–fetal transmission and consequent clinical manifestations such as hypoglycemia, hepatitis, and neonatal death[65]. As mentioned above, HEV genotype seems to be closely associated with the worse clinical outcome in pregnant women, since HEV3 does not generally cause death or fulminant hepatitis for pregnant women and the data are not available for the course of HEV4 infection during pregnancy[66,67]. As a possible mechanism of this fact, Gouilly et al[68] found that HEV1 proliferates more efficiently than HEV3 ex vivo in stromal cells and in tissue explants of decidua basalis and fetal placenta. They also demonstrated significant changes in the structure of the placental barrier with increased cellular death and necrosis at the maternal-fetal interface in HEV1-infected pregnant women. HEV1 also leads to the production of more infectious virions and pro-inflammatory cytokines like chemokines and IL-6. These changes in the cytokine microenvironment are associated with high viral load and an increase in tissue damage[68]. Additionally, significant changes occur in the immune system during pregnancy to protect the fetus from the maternal immune system. These alterations result in a shift from a T helper (Th)1-dominated immune response to a Th2-dominated one[69]. Furthermore, the function of the monocyte-macrophage system of pregnant women with acute liver failure is significantly impaired[70]. Nevertheless, the implications of these immunological alterations for the severity of acute HEV infections are not known yet. Lastly, pregnancy-related hormonal changes may also contribute to a poor outcome. The pregnant women with fulminant hepatic failure due to HEV infection have higher concentrations of estrogen, progesterone, and β-human chorionic gonadotrophin than HEV-negative pregnant women with fulminant hepatic failure or healthy controls[65]. Consistent with this, an in vitro study showed that serum from pregnant women, particularly those in the third trimester, amplified the HEV replication via inhibiting estrogen receptors and the synthesis of type I interferons[71]. As a result, the causal mechanisms of fatal courses in pregnancy remain unknown; thereby, further studies investigating the potential role of patients’ hormonal, immunological, and genetic factors and HEV variants should be performed to clarify the relationship between pregnancy and worse clinical outcomes in acute HEV1 and HEV2 infections.
ACLF
The typical manifestations of ACLF include acute worsening of the liver function with clinical complications such as the onset or deterioration of ascites and hepatic encephalopathy and coagulopathy. ACLF is associated with an elevated mortality level of nearly 70% in some reports[72,73]. A large prospective study including 343 patients with decompensated liver disease found that hepatic decompensations were associated with acute HEV infection in 11 patients, and three of these patients died[74]. HEV has been proposed as an overlooked cause of infectious trigger for ACLF. Manka et al[75] performed a retrospective analysis of 80 acute liver failure cases in a single center from Germany. Among all patients, eight had HEV RNA positivity together with supporting clinical findings of acute HEV infection, but half of them had an initially erroneous diagnosis of drug-induced liver injury. Another study from the United States also showed that a minority of suspected cases of drug-induced liver injury were actually caused by the HEV[76]. For the definition of ACLF, the European definition proposed by the European Association for the Study of the Liver-Chronic Liver Failure consortium was used through this article[77].
Chronic HEV infections in immunocompromised patients
Immunocompromised patients cannot achieve HEV clearance and may develop chronic hepatitis and cirrhosis if infected by HEV3 and HEV4[79,80]. In contrast, chronic HEV infection has not been observed in cases infected with HEV1 and HEV2 until now[78,79]. Although Kamar et al[78] described chronic HEV infections in liver and kidney transplant recipients for the first time in the literature in 2008, the exact definition of chronic HEV infection has been intensively debated for a long time. According to Kamar et al[59], solid organ transplant recipients who are viremic for more than 3 mo after the onset of HEV infection can be regarded as chronically infected and evaluated for treatment. However, spontaneous clearance without any HEV-specific treatment was demonstrated between 3 and 6 mo of the first detection of HEV viremia in a small number of solid-organ transplant recipients[80]. Importantly, performing the NAATs is mandatory in all patients for the identification of persistent HEV replication as a sign of chronic HEV infection since both anti-HEV IgG and IgM may remain negative under immunocompromised status[81].
Although fatigue is the most frequent symptom in chronic HEV infection, most patients have no symptom and only mild elevations in liver enzymes[81]. Nevertheless, chronic HEV infections may lead to structural injuries in the liver including nodules, fibrotic remodeling, and subsequent cirrhosis[82]. Approximately 20%-50% of transplant recipients having exposure with HEV3 develop chronic infection[83]. Within 2-5 years of chronic HEV infection, approximately 10% of patients develop cirrhosis[84]. Often, the progression in liver inflammation and injury can regress with HEV clearance[85]. The risk of the development of chronic HEV infection after HEV3 exposure is not associated with the magnitude of HEV load, but it is related with previous tacrolimus use and low lymphocyte count[71]. The clinical presentation of chronic HEV infection has mainly been described in the setting of solid-organ transplantation, but it can be similarly observed in other immunosuppressed patients including those with hematological malignancies undergoing chemotherapy[86-88], individuals with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome having low CD4+ cell count (< 200 cells/mm3)[89,90], and patients afflicted by rheumatic disorders and receiving immunosuppressive therapy[91].
Extrahepatic manifestations
HEV infections not only affect the liver but may also include other organ systems. Some disorders, including Guillain-Barre syndrome (GBS), neuralgic amyotrophy (NA), lymphoma, pancreatitis, thrombocytopenia, viral meningitis, thyroiditis, myocarditis, cryoglobulinemia, glomerulonephritis, Henoch-Schönlein purpura, and myasthenia gravis, have been claimed to be associated with HEV infection. Although some case series, animal models, and seroepidemiological studies supported casual relations between HEV infection and these extrahepatic manifestations, the exact underlying pathophysiological mechanisms have not yet been proven. Nevertheless, immune-mediated reactions and direct viral (cytopathic) tissue damage are the most commonly assumed culprit mechanisms in extrahepatic manifestations.
Neurological disorders: GBS is a typical neurological disorder emerging after some types of infections and causing severe damage to the peripheral nerves and nerve roots. Antibodies that are produced against gangliosides through molecular mimicry after culprit infections have been shown to lead to GBS[92]. GBS is one of the most frequently published extrahepatic complications of HEV infection, and it can occur after both acute and chronic infections of various HEV genotypes[93]. HEV-related GBS cases have been reported from both developed and developing countries. In a study from the United Kingdom and France, more than 5% of patients having HEV3 infection had neurological complications during follow-up[94]. In a case-control study, 10 out of 201 patients having GBS were infected by the HEV just before or at the onset of their illness[95]. The clinical features and outcomes in HEV-associated GBS resemble those in non-HEV-related GBS cases. A case-control study from Bangladesh reported that 11% (11 patients) of all patients who developed GBS had anti-HEV IgM positivity, and only one patient was HEV1 RNA positive. Of note, antibodies against gangliosides cannot be identified among these patients[96].
Besides GBS, facial nerve paralysis (Bell’s palsy), NA, polyradiculopathy, mononeuritis multiplex, viral meningitis, encephalitis, and myelitis are asserted as possibly related with HEV infection. NA (i.e. brachial neuritis) is another relatively frequent neurological manifestation of HEV infection and characterized by severe shoulder and arm pain, followed by weakness and atrophy[97]. The pathogenesis of NA is considered to be similar to that of GBS. Most patients presented with bilateral and severe involvement of the brachial plexus. In contrast to other causative agents, HEV-associated NA is not confined to the brachial plexus[98]. Other neurological presentations of HEV infections have been reported in case reports only.
Further studies should be performed to appreciate the actual pathophysiological mechanisms of neurological manifestations in HEV infection. Although some studies have demonstrated the ability of the HEV to complete the full viral life cycle in an oligodendrocyte[99], penetrate the blood-brain barrier[100], and stimulate mitochondrial apoptosis in HEV-infected gerbil brain tissues[101], it is unknown whether neurological manifestations are consequences of immune-mediated (mostly associated with molecular mimicry) mechanisms or the direct cytopathic effect of the HEV.
Hematological manifestations: Thrombocytopenia associated with HEV infections is generally not severe and does not require any specific treatment. However, severe thrombocytopenia was observed in nine patients who were infected by the HEV. Among these patients, the mean platelet count was 12 × 109/L[97]. The mechanism by which HEV infections lead to thrombocytopenia is not yet understood. It is considered being associated with the production of antiplatelet antibodies, as in most viral infections. However, only two out of nine patients had antibody against platelet in the aforementioned cases.
Hemolytic anemia was reported in patients with glucose-6-phosphate dehydrogenase deficiency having acute HEV infection[102,103]. In addition, aplastic anemia was reported in a young man from Pakistan[104]. However, more supporting data are needed to be confident about the causal relationship.
Acute pancreatitis: Acute pancreatitis is associated not only with the HEV but also with other hepatitis viruses (A, B, C). Most acute pancreatitis cases were reported from the Southern Asia region, suggesting that these were possibly caused by HEV1 infections[105,106]. HEV-associated acute pancreatitis is usually resolved with supportive care and mild to moderate in severity[106]. Although we do not have any strong evidence for the relation of HEV infection with acute pancreatitis, acute and severe epigastric pain should bring acute pancreatitis to mind as a possible differential diagnosis in patients having HEV infection. It is still unclear whether there is an increasing risk of acute pancreatitis after HEV3 and HEV4 infections.
Renal manifestations: The HEV can cause glomerulonephritis in both immuno-competent and immunosuppressed patients[107,108]. All glomerular diseases that have possible association with HEV infection were only reported in patients infected by HEV3, except one patient with HEV1 infection[108-110]. In total, at least eight patients with HEV3-associated glomerulonephritis have been described. The types of glomerulonephritis included membranoproliferative glomerulonephritis (n = 4), IgA-glomerulonephritis (n = 2), membranous nephropathy (n = 1), and nephroangio-sclerosis (n = 1). All except one glomerulonephritis case were identified in immunocompromised patients. HEV clearance achieved either by therapy or spontaneously improved renal functions and proteinuria levels among these cases[109,110]. Additionally, HEV RNA was detected in the cryoprecipitate obtained from one patient who developed cryoglobulinemic glomerulonephritis[108].
The underlying mechanism by which HEV infection may induce glomerular disease remains to be established. In HEV-induced glomerulonephritis, immune-mediated mechanisms are presumably playing an important role similar to hepatitis C virus (HCV)-associated glomerulonephritis in which immune complexes consisting of HCV antigen, anti-HCV IgG antibodies, and a rheumatoid factor accumulate in the glomerular tissue. To date, 10 cases of mixed cryoglobulinemia likely caused by HEV infection have been published[107-109,111]. All cases had type II or III mixed cryoglobulinemia and were considered to be caused by HEV3 since these occurred in HEV3 endemic regions. In mixed cryoglobulinemia, immunoglobulins that are insoluble at reduced temperatures cause injury in various tissues by accumulating in the vascular bed[97]. Cryoglobulinemia-associated glomerulonephritis is likely to be caused by an uncontrolled immune response to particular viral antigens, as seen in hepatitis B virus- or HCV-associated glomerulonephritis[107-109]. However, a direct cytopathic effect of the HEV on the glomeruli cannot be excluded.
Other manifestations: Other HEV-related extrahepatic manifestations have also been described. These include myocarditis, myositis, thyroiditis, Henoch-Schönlein purpura, and myasthenia gravis. However, more convincing data are needed to construct causality between these disorders and HEV infection.
TREATMENT
Treatment of acute HEV infection and ACLF
To date, no specific drugs have been approved for the treatment of HEV infections. Fortunately, in the vast majority of cases, acute HEV infection can be cleared spontaneously and does not require any specific treatment. However, acute HEV infection can progress to severe hepatitis and liver failure particularly in pregnant women and patients with underlying chronic liver diseases. In severe cases like ACLF, rapid clearance of the HEV and normalization of liver enzymes were noted with ribavirin treatment[112]. In these cases, there was a great variability in doses and durations of ribavirin treatment, and no severe adverse reaction related with ribavirin treatment[113]. Although there is no alternative treatment option to ribavirin in acute and severe hepatitis or ACLF caused by the HEV, the efficacy of ribavirin has still not been clarified by large-scale studies or randomized, controlled trials. In some case reports, corticosteroids were offered for slowing down the rate of progression to liver failure in patients with fulminant hepatitis E[78].
Current treatment options for pregnant women with HEV1- or HEV2-related severe hepatitis or liver failure are much more limited. Since ribavirin treatment is contraindicated in pregnant patients because of the teratogenic potential of ribavirin, no study has demonstrated the effectiveness of ribavirin in pregnant patients with severe hepatitis until now. However, Sinclair et al[114] reported no teratogenicity with ribavirin treatment for pregnant patients who were infected by the HCV and exposed to ribavirin directly or indirectly. This finding can be partly explained by the lack of teratogenic effects of ribavirin at the last trimester due to the completion of organogenesis in the first trimester of pregnancy. Therefore, ribavirin can be suggested for pregnant patients infected by the HEV in the last trimester of pregnancy in a case-by-case fashion considering the very high mortality rate (almost 20%) of HEV infection in that period. As an alternative approach, diligent follow-up of the liver function test and supportive care can be applied in treatment for pregnant patients infected by HEV1 or HEV2. Early liver transplantation should be considered in indicated patients[115]. However, the therapeutic termination of pregnancy cannot be recommended based on the current literature[116].
Treatment of chronic HEV infection
The first-line therapeutic approach for solid organ transplant recipients who have chronic HEV3 or HEV4 infection should be dose reduction of immunosuppressive medications, particularly those targeting T lymphocytes. This approach alone provides sustained viral clearance in up to one-third of patients[86]. Kamar et al[81] reported 25% (4/16) success rate in viral clearance only by reducing the doses of immuno-suppressive medications in solid-organ transplant recipients. However, all immunosuppressive drugs do not have the same effects. In in vitro studies, viral replication has been shown to be upregulated by mammalian target of rapamycin inhibitors but suppressed by mycophenolate mofetil[117,118]. It is essential to establish the clinical implications of these in vitro findings since chronic HEV infections have been reported in mycophenolate-receiving transplant patients.
Although pegylated interferon-alpha can be considered in liver transplant recipients and patients undergoing hemodialysis for the treatment of chronic HEV infection[118,119], it is contraindicated in renal, pancreas, heart, and lung transplant patients because of enhanced immune response and the risk of rejection[120]. Therefore, ribavirin constitutes the treatment of choice in chronic HEV infections in many solid-organ transplant recipients, despite that its efficacy has not been endorsed by randomized, controlled trials. In a multicenter retrospective study including 59 solid organ transplant recipients treated with ribavirin at a median dose of 600 (range, 29-1200) mg/d for 3 (range, 1-18) mo, the rate of sustained virologic response (undetectable HEV RNA in serum 6 mo after the completion of ribavirin treatment) was 78%. Additionally, one-third of patients with persistent HEV viremia at the end of the 3-mo treatment achieved sustained virologic response after receiving ribavirin for a longer period[121]. Kamar et al[122] collected data retrospectively from 30 European centers to depict the outcomes of ribavirin treatment among 255 solid-organ transplant recipients with chronic HEV3 infection. The primary aim of this study was to describe the ribavirin treatment responses and determinants of sustained virologic response. In the results of this study, 81% of all patients achieved sustained virologic response with an initial ribavirin treatment at a median dose of 600 mg/d for a median duration of 3 mo, while the rate of sustained virologic response elevated to 90% when an additional course of ribavirin was given to those who did not initially achieve sustained virologic response. This study also confirmed that higher baseline lymphocyte count and good hematologic tolerance to ribavirin (i.e., not needing ribavirin dose reduction) were predictors of achieving sustained virologic response. Nevertheless, tolerability of ribavirin treatment is still a major concern. In this study, 28% of patients needed ribavirin dose adjustment because of hematological side effects. Although the optimal duration of ribavirin therapy remains unclear, the 3-mo treatment is the most widely used treatment modality for chronic HEV infections. At the completion of ribavirin treatment, the detection of HEV RNA in the stools of patients in whom HEV RNA was negative in the serum was reported as being associated with an increased risk of HEV viremia at follow-up[123]. Also, Kamar et al[124] showed that a reduction in the HEV RNA concentration of ≥ 0.5 log10 IU/mL at day 7 was a highly predictive marker of sustained virologic response. In the same study, the serum ribavirin trough level had no impact on sustained virologic response at the 7th or 60th d of therapy.
Chronic HEV infection can be treated by pegylated interferon-alpha, ribavirin, or the combination of two drugs in non-transplant immunosuppressed patients, that is patients with hematological disorders or HIV, according to the results of a few case reports and small case series[88,125-127]. In line with these findings, Tavitian et al[128] reported that 75% (9/12) of stem cell transplant recipients achieved sustained virologic response with ribavirin treatment.
The antiviral mechanism of ribavirin against the HEV is not completely understood. Ribavirin seems to inhibit HEV replication by depleting guanosine triphosphate pools, which probably inhibits inosine monophosphate dehydrogenase and prevents HEV RNA replication[129]. However, the deep sequences revealed G1634R and Y1320H mutations in the HEV RNA polymerase gene in patients who cannot achieve sustained virologic response with ribavirin treatment[130,131]. Furthermore, the G1634R mutation was found to emerge during ribavirin treatment in relapsed patients[130]. Interestingly, some studies showed that prolong treatment with ribavirin (e.g., 6 mo duration) can achieve sustained virologic response even in HEV infections that cannot be cured by 3-mo ribavirin therapy and caused by HEV strains carrying the G1634R mutation[132]. Additional mutational variants in the HEV polymerase gene have been subsequently described. Interestingly, some of these mutations render the HEV sensitive to ribavirin; others promote or suppress HEV replication[131,133]. Hence, the role of HEV RNA polymerase mutations and their effects on HEV infection-related outcomes are not yet established.
Ribavirin treatment can cause some side effects including skin reactions, dose-dependent hemolytic anemia, and dry cough. As patients with chronic HEV infection have some comorbidities resulting in impaired renal function or anemia, ribavirin doses should be adjusted cautiously in these patients[134].
Treatment of extrahepatic complications
The extrahepatic manifestations of HEV infections can be treated by either ribavirin or immunosuppressive medications such as corticosteroids. Before deciding treatment for these manifestations, the main mechanism of extrahepatic manifestation in question should first be determined. As mentioned before, extrahepatic manifestations are mainly mediated by immunological mechanisms or the direct viral (cytopathic) effect of the HEV. Therefore, treatment (ribavirin or immunosuppressive drugs) should be chosen according to the main pathophysiological mechanisms of extrahepatic manifestations.
PROMISING TREATMENT OPTIONS FOR RIBAVIRIN-RESISTANT HEV INFECTIONS
Sofosbuvir, an NS5B polymerase inhibitor, was approved by the Food and Drug Administration for the treatment of hepatitis C. Because of its high in vitro efficacy, sofosbuvir was also considered as an option in the treatment of ribavirin-resistant HEV infections[135]. However, a study involving 10 cases with chronic HEV infection reported only partial response and high rate of relapse with sofosbuvir monotherapy[136]. Moreover, it was administered as combination therapy with ribavirin in some ribavirin-resistant cases. Biliotti et al[137] showed the clearance of the HEV with sofosbuvir/ribavirin combination therapy in patients with acute HEV infection. In another study, combination therapy was reported to treat refractory HEV infection in an immunosuppressed individual[138]. In contrast, other studies demonstrated that sofosbuvir/ribavirin combination therapy was not able to provide sustained virologic response in chronic HEV infections observed in solid-organ transplant recipients and patients infected by the HIV[139-141]. Similarly, Schulz et al[142] reported that sustained virologic response could not be achieved with ribavirin and sofosbuvir combination in a multi-organ transplanted patient with chronic HEV3 infection. In the deep sequence analysis undertaken at three separate time points, a stepwise accumulation of four well-characterized ribavirin-associated resistance mutations (K1383N, D1384N, V1479I, and G1634R) was identified. The results of an ongoing phase 2 clinical trial are curiously awaited to understand the efficacy of sofosbuvir in the treatment of HEV infection.
Nishiyama et al[143] reported a promising compound, 2'-C-methylguanosine, that suppressed the growth of HEV3 in cell cultures and showed in vitro synergistic interaction with ribavirin against the HEV. However, there is no study yet exploring its efficacy and safety in animal models and human trials.
Zinc can be a potential adjuvant therapy in ribavirin-resistant and/or relapsed HEV infections. Horvatits et al[144] reported the favorable in vitro efficacy of zinc in a patient with a breakthrough HEV infection under 800 mg/d of ribavirin treatment[145]. Additionally, authors identified significantly lower serum zinc level in patients with chronic HEV infection than in the control group[144]. More importantly, the addition of 120 mg/d of zinc to existing ribavirin treatment cleared the HEV in this patient[144]. In contrast, two solid-organ transplant recipients with chronic HEV infections failed to achieve sustained virologic response with ribavirin treatment despite having elevated intra-erythrocyte zinc concentrations[145]. Clearly, further large-scale studies are required to understand the impact of adjuvant zinc therapy on patients who have chronic HEV infection and fail to achieve sustained virologic response under ribavirin monotherapy.
In vitro, the natural compound silvestrol has an inhibitory effect on HEV replication[146]. Additionally, silvestrol-treated mice showed a rapid diminish in fecal concentrations of HEV RNA[146]. However, it has not yet been tested on humans.
NITD008 and GPC-N114 were originally developed to treat the dengue virus and picornaviruses, respectively. These two novel antiviral candidates demonstrated a potent inhibitory effect against HEV replication without causing significant cellular cytotoxicity in cell cultures[147]. However, the antiviral efficacy and safety of these compounds are still unknown for HEV infections in humans.
VACCINE
In 2010, an HEV vaccine, based on a protein encoded by ORF 2 of an HEV1, was assessed in a phase 3 trial including more than 100000 participants from China[148]. In this phase 3 trial, the long-term efficacy and safety of this vaccine was explored over more than 4 years in a vaccinated group (n = 56302 participants) in comparison with a control group (n = 56302 participants). The authors of this trial identified only 60 cases of hepatitis E, and seven of them belonged to the vaccinated group. Furthermore, no serious adverse events related to the vaccine were observed[148]. Because of the endemicity of HEV1 and HEV4 in China, the protective effect of this vaccine could be assumed for HEV1 and four infections, but these findings cannot be extrapolated for HEV3 infections. That is why the National Institute of Health decided to perform a phase 1 trial to investigate the safety of this vaccine, and phase 2 and 3 trials will likely follow that trial. Therefore, the findings from these trials will demonstrate the safety and efficacy of HEV vaccine (Hecolin) in an HEV3 endemic region. Additionally, an ongoing large trial is testing Hecolin in more than 20000 pregnant women in Bangladesh. The results of this study would be quite important to understand the effectiveness and safety of Hecolin in pregnant women (clinicaltrials.gov, NCT02759991) who are under great risk of HEV1 infections.
CONCLUSION
The HEV is an important cause of viral hepatitis in both high- and low-income countries. Previously, HEV infections were considered a problem of undeveloped countries, which have poor sanitation standards and clean drinking water supply systems. The recent data revealed that the global burden of HEV infection is greater than previously estimated, and autochthonous HEV infections in high-income countries are prevailing worldwide. Additionally, the epidemiology of HEV infections has not yet been established in many countries. The great majority of HEV infections are asymptomatic or mildly symptomatic, although pregnant women and patients with chronic liver diseases have a significant risk of severe hepatitis and hepatic failure. Furthermore, immunocompromised patients may develop chronic hepatitis after HEV3 or HEV4 exposure. Besides hepatic manifestations, HEV infections may lead to diverse extrahepatic involvements such as neurological and renal manifestations. Although ribavirin has been used in the treatment of chronic HEV infections for several years, there is no randomized, controlled trial that supports the safety and efficacy of ribavirin in the treatment of HEV infections. Similarly, there is no robust evidence for the effectiveness of ribavirin and interferon-alpha therapies in acute HEV infection. Moreover, ribavirin is not a panacea, and the administration of ribavirin for pregnant women is highly debatable. Therefore, new compounds that are safe and effective are needed for patients having ribavirin intolerance and infected by the ribavirin-resistant HEV.
Footnotes
Conflict-of-interest statement: All authors have no conflicts of interest to be stated.
Manuscript source: Invited manuscript
Peer-review started: June 28, 2020
First decision: July 28, 2020
Article in press: September 10, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Parvez MK, Pineau P, Shim J S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Abdullah Tarık Aslan, Department of Internal Medicine, Gölhisar State Hospital, Burdur 15100, Turkey.
Hatice Yasemin Balaban, Department of Gastroenterology, Hacettepe University Faculty of Medicine, Ankara 06100, Turkey. yhbalaban@gmail.com.
References
- 1.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 2.Krawczynski K. Foreword. Hepatitis E virus. Semin Liver Dis. 2013;33:1–2. doi: 10.1055/s-0033-1338119. [DOI] [PubMed] [Google Scholar]
- 3.Chandra NS, Sharma A, Malhotra B, Rai RR. Dynamics of HEV viremia, fecal shedding and its relationship with transaminases and antibody response in patients with sporadic acute hepatitis E. Virol J. 2010;7:213. doi: 10.1186/1743-422X-7-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra JAAA, Kampa KC, Morsoletto DGB, Junior AP, Ivantes CAP. Hepatitis E: A Literature Review. J Clin Transl Hepatol. 2017;5:376–383. doi: 10.14218/JCTH.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 6.Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, Bradley DW. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 7.Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry KE, Tam AW, Smith MM, Kim JP, Luk KC, Young LM, Piatak M, Feldman RA, Yun KY, Purdy MA. Hepatitis E virus (HEV): strain variation in the nonstructural gene region encoding consensus motifs for an RNA-dependent RNA polymerase and an ATP/GTP binding site. Virus Genes. 1992;6:173–185. doi: 10.1007/BF01703066. [DOI] [PubMed] [Google Scholar]
- 9.Rozanov MN, Koonin EV, Gorbalenya AE. Conservation of the putative methyltransferase domain: a hallmark of the 'Sindbis-like' supergroup of positive-strand RNA viruses. J Gen Virol. 1992;73(Pt 8):2129–2134. doi: 10.1099/0022-1317-73-8-2129. [DOI] [PubMed] [Google Scholar]
- 10.Jameel S, Zafrullah M, Ozdener MH, Panda SK. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J Virol. 1996;70:207–216. doi: 10.1128/jvi.70.1.207-216.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Q, Heller B, Capuccino JM, Song B, Nimgaonkar I, Hrebikova G, Contreras JE, Ploss A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc Natl Acad Sci USA. 2017;114:1147–1152. doi: 10.1073/pnas.1614955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair VP, Anang S, Subramani C, Madhvi A, Bakshi K, Srivastava A, Shalimar, Nayak B, Ranjith Kumar CT, Surjit M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS Pathog. 2016;12:e1005521. doi: 10.1371/journal.ppat.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing L, Wang JC, Li TC, Yasutomi Y, Lara J, Khudyakov Y, Schofield D, Emerson SU, Purcell RH, Takeda N, Miyamura T, Cheng RH. Spatial configuration of hepatitis E virus antigenic domain. J Virol. 2011;85:1117–1124. doi: 10.1128/JVI.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, Zhang J, Tao YJ. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci USA. 2009;106:12992–12997. doi: 10.1073/pnas.0904848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimgaonkar I, Ding Q, Schwartz RE, Ploss A. Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol. 2018;15:96–110. doi: 10.1038/nrgastro.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holla RP, Ahmad I, Ahmad Z, Jameel S. Molecular virology of hepatitis E virus. Semin Liver Dis. 2013;33:3–14. doi: 10.1055/s-0033-1338110. [DOI] [PubMed] [Google Scholar]
- 17.Dai X, Dong C, Zhou Z, Liang J, Dong M, Yang Y, Fu J, Tian H, Wang S, Fan J, Meng J, Purdy MA. Hepatitis E virus genotype 4, Nanjing, China, 2001-2011. Emerg Infect Dis. 2013;19:1528–1530. doi: 10.3201/eid1909.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal R. Hepatitis E: Historical, contemporary and future perspectives. J Gastroenterol Hepatol. 2011;26 Suppl 1:72–82. doi: 10.1111/j.1440-1746.2010.06540.x. [DOI] [PubMed] [Google Scholar]
- 19.VISWANATHAN R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res. 1957;45:145–155. [PubMed] [Google Scholar]
- 20.Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 21.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 22.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 23.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat. 2009;16:519–523. doi: 10.1111/j.1365-2893.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 25.Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–784. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- 26.Doceul V, Bagdassarian E, Demange A, Pavio N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses. 2016;8 doi: 10.3390/v8100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlosser J, Eiden M, Vina-Rodriguez A, Fast C, Dremsek P, Lange E, Ulrich RG, Groschup MH. Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet Res. 2014;45:121. doi: 10.1186/s13567-014-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izopet J, Dubois M, Bertagnoli S, Lhomme S, Marchandeau S, Boucher S, Kamar N, Abravanel F, Guérin JL. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg Infect Dis. 2012;18:1274–1281. doi: 10.3201/eid1808.120057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B, Zhang L, Gong L, Lv J, Feng Y, Liu J, Song L, Xu Q, Jiang M, Xu A. Hepatitis E Virus in Yellow Cattle, Shandong, Eastern China. Emerg Infect Dis. 2016;22:2211–2212. doi: 10.3201/eid2212.160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang F, Li Y, Yu W, Jing S, Wang J, Long F, He Z, Yang C, Bi Y, Cao W, Liu C, Hua X, Pan Q. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology. 2016;64:350–359. doi: 10.1002/hep.28668. [DOI] [PubMed] [Google Scholar]
- 31.Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153–5159. doi: 10.1128/AEM.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terio V, Bottaro M, Pavoni E, Losio MN, Serraino A, Giacometti F, Martella V, Mottola A, Di Pinto A, Tantillo G. Occurrence of hepatitis A and E and norovirus GI and GII in ready-to-eat vegetables in Italy. Int J Food Microbiol. 2017;249:61–65. doi: 10.1016/j.ijfoodmicro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Maunula L, Kaupke A, Vasickova P, Söderberg K, Kozyra I, Lazic S, van der Poel WH, Bouwknegt M, Rutjes S, Willems KA, Moloney R, D'Agostino M, de Roda Husman AM, von Bonsdorff CH, Rzeżutka A, Pavlik I, Petrovic T, Cook N. Tracing enteric viruses in the European berry fruit supply chain. Int J Food Microbiol. 2013;167:177–185. doi: 10.1016/j.ijfoodmicro.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Riveiro-Barciela M, Sauleda S, Quer J, Salvador F, Gregori J, Pirón M, Rodríguez-Frías F, Buti M. Red blood cell transfusion-transmitted acute hepatitis E in an immunocompetent subject in Europe: a case report. Transfusion. 2017;57:244–247. doi: 10.1111/trf.13876. [DOI] [PubMed] [Google Scholar]
- 35.Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 36.Nicaise G, Gillot I, Julliard AK, Keicher E, Blaineau S, Amsellem J, Meyran JC, Hernandez-Nicaise ML, Ciapa B, Gleyzal C. X-ray microanalysis of calcium containing organelles in resin embedded tissue. Scanning Microsc. 1989;3:199–219; discussion 219-220. [PubMed] [Google Scholar]
- 37.Ankcorn MJ, Tedder RS, Cairns J, Sandmann FG. Cost-Effectiveness Analysis of Screening for Persistent Hepatitis E Virus Infection in Solid Organ Transplant Patients in the United Kingdom: A Model-Based Economic Evaluation. Value Health. 2020;23:309–318. doi: 10.1016/j.jval.2019.09.2751. [DOI] [PubMed] [Google Scholar]
- 38.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 39.Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56:500–502. doi: 10.1016/j.jhep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 41.Mansuy JM, Bendall R, Legrand-Abravanel F, Sauné K, Miédouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–2312. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aspinall EJ, Couturier E, Faber M, Said B, Ijaz S, Tavoschi L, Takkinen J, Adlhoch C The Country Experts. Hepatitis E virus infection in Europe: surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.26.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doting MHE, Weel J, Niesters HGM, Riezebos-Brilman A, Brandenburg A. The added value of hepatitis E diagnostics in determining causes of hepatitis in routine diagnostic settings in the Netherlands. Clin Microbiol Infect. 2017;23:667–671. doi: 10.1016/j.cmi.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Ditah I, Ditah F, Devaki P, Ditah C, Kamath PS, Charlton M. Current epidemiology of hepatitis E virus infection in the United States: low seroprevalence in the National Health and Nutrition Evaluation Survey. Hepatology. 2014;60:815–822. doi: 10.1002/hep.27219. [DOI] [PubMed] [Google Scholar]
- 45.Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. doi: 10.1038/nrdp.2017.86. [DOI] [PubMed] [Google Scholar]
- 46.Li TC, Kataoka M, Takahashi K, Yoshizaki S, Kato T, Ishii K, Takeda N, Mishiro S, Wakita T. Generation of hepatitis E virus-like particles of two new genotypes G5 and G6 and comparison of antigenic properties with those of known genotypes. Vet Microbiol. 2015;178:150–157. doi: 10.1016/j.vetmic.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Rasche A, Saqib M, Liljander AM, Bornstein S, Zohaib A, Renneker S, Steinhagen K, Wernery R, Younan M, Gluecks I, Hilali M, Musa BE, Jores J, Wernery U, Drexler JF, Drosten C, Corman VM. Hepatitis E Virus Infection in Dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983-2015. Emerg Infect Dis. 2016;22:1249–1252. doi: 10.3201/eid2207.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355–7.e3. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 49.Sridhar S, Yip CC, Wu S, Chew NF, Leung KH, Chan JF, Zhao PS, Chan WM, Poon RW, Tsoi HW, Cai JP, Chan HS, Leung AW, Tse CW, Zee JS, Tsang OT, Cheng VC, Lau SK, Woo PC, Tsang DN, Yuen KY. Transmission of rat hepatitis E virus infection to humans in Hong Kong: a clinical and epidemiological analysis. Hepatology. 2020 doi: 10.1002/hep.31138. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Waterborne outbreaks of hepatitis E: recognition, investigation and control. Technical report. 2014. Available from: https://www.who.int/hiv/pub/hepatitis/HepE-manual/en/ [Google Scholar]
- 51.Abravanel F, Chapuy-Regaud S, Lhomme S, Miedougé M, Peron JM, Alric L, Rostaing L, Kamar N, Izopet J. Performance of anti-HEV assays for diagnosing acute hepatitis E in immunocompromised patients. J Clin Virol. 2013;58:624–628. doi: 10.1016/j.jcv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Legrand-Abravanel F, Thevenet I, Mansuy JM, Saune K, Vischi F, Peron JM, Kamar N, Rostaing L, Izopet J. Good performance of immunoglobulin M assays in diagnosing genotype 3 hepatitis E virus infections. Clin Vaccine Immunol. 2009;16:772–774. doi: 10.1128/CVI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang S, Zhang X, Jiang H, Yan Q, Ai X, Wang Y, Cai J, Jiang L, Wu T, Wang Z, Guan L, Shih JW, Ng MH, Zhu F, Zhang J, Xia N. Profile of acute infectious markers in sporadic hepatitis E. PLoS One. 2010;5:e13560. doi: 10.1371/journal.pone.0013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–1271. doi: 10.1016/j.jhep.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Abravanel F, Lhomme S, Chapuy-Regaud S, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Kamar N, Izopet J. Hepatitis E virus reinfections in solid-organ-transplant recipients can evolve into chronic infections. J Infect Dis. 2014;209:1900–1906. doi: 10.1093/infdis/jiu032. [DOI] [PubMed] [Google Scholar]
- 56.Wen GP, Tang ZM, Yang F, Zhang K, Ji WF, Cai W, Huang SJ, Wu T, Zhang J, Zheng ZZ, Xia NS. A valuable antigen detection method for diagnosis of acute hepatitis E. J Clin Microbiol. 2015;53:782–788. doi: 10.1128/JCM.01853-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao C, Geng Y, Harrison TJ, Huang W, Song A, Wang Y. Evaluation of an antigen-capture EIA for the diagnosis of hepatitis E virus infection. J Viral Hepat. 2015;22:957–963. doi: 10.1111/jvh.12397. [DOI] [PubMed] [Google Scholar]
- 58.Behrendt P, Bremer B, Todt D, Brown RJ, Heim A, Manns MP, Steinmann E, Wedemeyer H. Hepatitis E Virus (HEV) ORF2 Antigen Levels Differentiate Between Acute and Chronic HEV Infection. J Infect Dis. 2016;214:361–368. doi: 10.1093/infdis/jiw161. [DOI] [PubMed] [Google Scholar]
- 59.Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935–1936. doi: 10.1111/ajt.12253. [DOI] [PubMed] [Google Scholar]
- 60.Baylis SA, Blümel J, Mizusawa S, Matsubayashi K, Sakata H, Okada Y, Nübling CM, Hanschmann KM HEV Collaborative Study Group. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg Infect Dis. 2013;19:729–735. doi: 10.3201/eid1905.121845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Lhomme S, Marion O, Abravanel F, Izopet J, Kamar N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J Clin Med. 2020;9 doi: 10.3390/jcm9020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pischke S, Wedemeyer H. Hepatitis E virus infection: multiple faces of an underestimated problem. J Hepatol. 2013;58:1045–1046. doi: 10.1016/j.jhep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 64.NAIDU SS, VISWANATHAN R. Infectious hepatitis in pregnancy during Delhi epidemic. Indian J Med Res. 1957;45:71–76. [PubMed] [Google Scholar]
- 65.Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 66.Anty R, Ollier L, Péron JM, Nicand E, Cannavo I, Bongain A, Giordanengo V, Tran A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J Clin Virol. 2012;54:76–78. doi: 10.1016/j.jcv.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Bouthry E, Benachi A, Vivanti AJ, Letamendia E, Vauloup-Fellous C, Roque-Afonso AM. Autochthonous Hepatitis E during Pregnancy, France. Emerg Infect Dis. 2018;24:1586–1587. doi: 10.3201/eid2408.180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gouilly J, Chen Q, Siewiera J, Cartron G, Levy C, Dubois M, Al-Daccak R, Izopet J, Jabrane-Ferrat N, El Costa H. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface. Nat Commun. 2018;9:4748. doi: 10.1038/s41467-018-07200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 70.Sehgal R, Patra S, David P, Vyas A, Khanam A, Hissar S, Gupta E, Kumar G, Kottilil S, Maiwall R, Sarin SK, Trehanpati N. Impaired monocyte-macrophage functions and defective Toll-like receptor signaling in hepatitis E virus-infected pregnant women with acute liver failure. Hepatology. 2015;62:1683–1696. doi: 10.1002/hep.28143. [DOI] [PubMed] [Google Scholar]
- 71.Bi Y, Yang C, Yu W, Zhao X, Zhao C, He Z, Jing S, Wang H, Huang F. Pregnancy serum facilitates hepatitis E virus replication in vitro. J Gen Virol. 2015;96:1055–1061. doi: 10.1099/vir.0.000054. [DOI] [PubMed] [Google Scholar]
- 72.Péron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298–303. doi: 10.1111/j.1365-2893.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 73.Kumar A, Saraswat VA. Hepatitis E and Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2013;3:225–230. doi: 10.1016/j.jceh.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blasco-Perrin H, Madden RG, Stanley A, Crossan C, Hunter JG, Vine L, Lane K, Devooght-Johnson N, Mclaughlin C, Petrik J, Stableforth B, Hussaini H, Phillips M, Mansuy JM, Forrest E, Izopet J, Blatchford O, Scobie L, Peron JM, Dalton HR. Hepatitis E virus in patients with decompensated chronic liver disease: a prospective UK/French study. Aliment Pharmacol Ther. 2015;42:574–581. doi: 10.1111/apt.13309. [DOI] [PubMed] [Google Scholar]
- 75.Manka P, Bechmann LP, Coombes JD, Thodou V, Schlattjan M, Kahraman A, Syn WK, Saner F, Gerken G, Baba H, Verheyen J, Timm J, Canbay A. Hepatitis E Virus Infection as a Possible Cause of Acute Liver Failure in Europe. Clin Gastroenterol Hepatol. 2015;13:1836–1842.e2; quiz e157-158. doi: 10.1016/j.cgh.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH, Tillmann HL, Gu J, Serrano J, Hoofnagle JH Drug-Induced Liver Injury Network (DILIN) Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–72.e1-9. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 78.Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 79.Geng Y, Zhang H, Huang W, J Harrison T, Geng K, Li Z, Wang Y. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat Mon. 2014;14:e15618. doi: 10.5812/hepatmon.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meisner S, Polywka S, Memmler M, Nashan B, Lohse AW, Sterneck M, Pischke S. Definition of chronic hepatitis E after liver transplant conforms to convention. Am J Transplant. 2015;15:3011–3012. doi: 10.1111/ajt.13428. [DOI] [PubMed] [Google Scholar]
- 81.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 82.Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859–860. doi: 10.1056/NEJMc0708687. [DOI] [PubMed] [Google Scholar]
- 83.Pischke S, Stiefel P, Franz B, Bremer B, Suneetha PV, Heim A, Ganzenmueller T, Schlue J, Horn-Wichmann R, Raupach R, Darnedde M, Scheibner Y, Taubert R, Haverich A, Manns MP, Wedemeyer H, Bara CL. Chronic hepatitis e in heart transplant recipients. Am J Transplant. 2012;12:3128–3133. doi: 10.1111/j.1600-6143.2012.04200.x. [DOI] [PubMed] [Google Scholar]
- 84.Kamar N, Mansuy JM, Cointault O, Selves J, Abravanel F, Danjoux M, Otal P, Esposito L, Durand D, Izopet J, Rostaing L. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8:1744–1748. doi: 10.1111/j.1600-6143.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- 85.Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, Cointault O, Ribes D, Cardeau I, Nogier MB, Mansuy JM, Muscari F, Peron JM, Izopet J, Rostaing L. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353–360. doi: 10.1097/TP.0b013e3181c4096c. [DOI] [PubMed] [Google Scholar]
- 86.Péron JM, Mansuy JM, Récher C, Bureau C, Poirson H, Alric L, Izopet J, Vinel JP. Prolonged hepatitis E in an immunocompromised patient. J Gastroenterol Hepatol. 2006;21:1223–1224. doi: 10.1111/j.1440-1746.2006.04209.x. [DOI] [PubMed] [Google Scholar]
- 87.Tamura A, Shimizu YK, Tanaka T, Kuroda K, Arakawa Y, Takahashi K, Mishiro S, Shimizu K, Moriyama M. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–120. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 88.Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann Intern Med. 2010;153:135–136. doi: 10.7326/0003-4819-153-2-201007200-00256. [DOI] [PubMed] [Google Scholar]
- 89.Colson P, Kaba M, Moreau J, Brouqui P. Hepatitis E in an HIV-infected patient. J Clin Virol. 2009;45:269–271. doi: 10.1016/j.jcv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Kenfak-Foguena A, Schöni-Affolter F, Bürgisser P, Witteck A, Darling KE, Kovari H, Kaiser L, Evison JM, Elzi L, Gurter-De La Fuente V, Jost J, Moradpour D, Abravanel F, Izpopet J, Cavassini M Data Center of the Swiss HIV Cohort Study, Lausanne, Switzerland. Hepatitis E Virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17:1074–1078. doi: 10.3201/eid1706.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pischke S, Peron JM, von Wulffen M, von Felden J, Höner Zu Siederdissen C, Fournier S, Lütgehetmann M, Iking-Konert C, Bettinger D, Par G, Thimme R, Cantagrel A, Lohse AW, Wedemeyer H, de Man R, Mallet V. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses. 2019;11 doi: 10.3390/v11020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 93.Dalton HR, Kamar N, van Eijk JJ, Mclean BN, Cintas P, Bendall RP, Jacobs BC. Hepatitis E virus and neurological injury. Nat Rev Neurol. 2016;12:77–85. doi: 10.1038/nrneurol.2015.234. [DOI] [PubMed] [Google Scholar]
- 94.Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S, Izopet J, Dalton HR. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173–179. doi: 10.3201/eid1702.100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Berg B, van der Eijk AA, Pas SD, Hunter JG, Madden RG, Tio-Gillen AP, Dalton HR, Jacobs BC. Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82:491–497. doi: 10.1212/WNL.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 96.Geurtsvankessel CH, Islam Z, Mohammad QD, Jacobs BC, Endtz HP, Osterhaus AD. Hepatitis E and Guillain-Barre syndrome. Clin Infect Dis. 2013;57:1369–1370. doi: 10.1093/cid/cit512. [DOI] [PubMed] [Google Scholar]
- 97.Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082–1095. doi: 10.1016/j.jhep.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 98.van Eijk JJJ, Dalton HR, Ripellino P, Madden RG, Jones C, Fritz M, Gobbi C, Melli G, Pasi E, Herrod J, Lissmann RF, Ashraf HH, Abdelrahim M, Masri OABAL, Fraga M, Benninger D, Kuntzer T, Aubert V, Sahli R, Moradpour D, Blasco-Perrin H, Attarian S, Gérolami R, Colson P, Giordani MT, Hartl J, Pischke S, Lin NX, Mclean BN, Bendall RP, Panning M, Peron JM, Kamar N, Izopet J, Jacobs BC, van Alfen N, van Engelen BGM. Clinical phenotype and outcome of hepatitis E virus-associated neuralgic amyotrophy. Neurology. 2017;89:909–917. doi: 10.1212/WNL.0000000000004297. [DOI] [PubMed] [Google Scholar]
- 99.Drave SA, Debing Y, Walter S, Todt D, Engelmann M, Friesland M, Wedemeyer H, Neyts J, Behrendt P, Steinmann E. Extra-hepatic replication and infection of hepatitis E virus in neuronal-derived cells. J Viral Hepat. 2016;23:512–521. doi: 10.1111/jvh.12515. [DOI] [PubMed] [Google Scholar]
- 100.Shi R, Soomro MH, She R, Yang Y, Wang T, Wu Q, Li H, Hao W. Evidence of Hepatitis E virus breaking through the blood-brain barrier and replicating in the central nervous system. J Viral Hepat. 2016;23:930–939. doi: 10.1111/jvh.12557. [DOI] [PubMed] [Google Scholar]
- 101.Tian J, Shi R, Xiao P, Liu T, She R, Wu Q, An J, Hao W, Soomro M. Hepatitis E Virus Induces Brain Injury Probably Associated With Mitochondrial Apoptosis. Front Cell Infect Microbiol. 2019;9:433. doi: 10.3389/fcimb.2019.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zamvar V, McClean P, Odeka E, Richards M, Davison S. Hepatitis E virus infection with nonimmune hemolytic anemia. J Pediatr Gastroenterol Nutr. 2005;40:223–225. doi: 10.1097/00005176-200502000-00027. [DOI] [PubMed] [Google Scholar]
- 103.Monga A, Makkar RP, Arora A, Mukhopadhyay S, Gupta AK. Case report: Acute hepatitis E infection with coexistent glucose-6-phosphate dehydrogenase deficiency. Can J Infect Dis. 2003;14:230–231. doi: 10.1155/2003/913679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah SA, Lal A, Idrees M, Hussain A, Jeet C, Malik FA, Iqbal Z, Rehman Hu. Hepatitis E virus-associated aplastic anaemia: the first case of its kind. J Clin Virol. 2012;54:96–97. doi: 10.1016/j.jcv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Mishra A, Saigal S, Gupta R, Sarin SK. Acute pancreatitis associated with viral hepatitis: a report of six cases with review of literature. Am J Gastroenterol. 1999;94:2292–2295. doi: 10.1111/j.1572-0241.1999.01318.x. [DOI] [PubMed] [Google Scholar]
- 106.Bazerbachi F, Haffar S, Garg SK, Lake JR. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf) 2016;4:1–15. doi: 10.1093/gastro/gov042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, Esposito L, Cardeau-Desangles I, Guitard J, Sallusto F, Muscari F, Peron JM, Alric L, Izopet J, Rostaing L. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617–623. doi: 10.1097/TP.0b013e318245f14c. [DOI] [PubMed] [Google Scholar]
- 108.Guinault D, Ribes D, Delas A, Milongo D, Abravanel F, Puissant-Lubrano B, Izopet J, Kamar N. Hepatitis E Virus-Induced Cryoglobulinemic Glomerulonephritis in a Nonimmunocompromised Person. Am J Kidney Dis. 2016;67:660–663. doi: 10.1053/j.ajkd.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 109.Del Bello A, Guilbeau-Frugier C, Josse AG, Rostaing L, Izopet J, Kamar N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl Infect Dis. 2015;17:279–283. doi: 10.1111/tid.12353. [DOI] [PubMed] [Google Scholar]
- 110.Taton B, Moreau K, Lepreux S, Bachelet T, Trimoulet P, De Ledinghen V, Pommereau A, Ronco P, Kamar N, Merville P, Couzi L. Hepatitis E virus infection as a new probable cause of de novo membranous nephropathy after kidney transplantation. Transpl Infect Dis. 2013;15:E211–E215. doi: 10.1111/tid.12143. [DOI] [PubMed] [Google Scholar]
- 111.Pischke S, Behrendt P, Manns MP, Wedemeyer H. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect Dis. 2014;14:678–679. doi: 10.1016/S1473-3099(14)70823-0. [DOI] [PubMed] [Google Scholar]
- 112.Péron JM, Dalton H, Izopet J, Kamar N. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol. 2011;54:1323–4; author reply 1324-1325. doi: 10.1016/j.jhep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Pischke S, Hardtke S, Bode U, Birkner S, Chatzikyrkou C, Kauffmann W, Bara CL, Gottlieb J, Wenzel J, Manns MP, Wedemeyer H. Ribavirin treatment of acute and chronic hepatitis E: a single-centre experience. Liver Int. 2013;33:722–726. doi: 10.1111/liv.12114. [DOI] [PubMed] [Google Scholar]
- 114.Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC. The Ribavirin Pregnancy Registry: An Interim Analysis of Potential Teratogenicity at the Mid-Point of Enrollment. Drug Saf. 2017;40:1205–1218. doi: 10.1007/s40264-017-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kar P, Sengupta A. A guide to the management of hepatitis E infection during pregnancy. Expert Rev Gastroenterol Hepatol. 2019;13:205–211. doi: 10.1080/17474124.2019.1568869. [DOI] [PubMed] [Google Scholar]
- 116.Zhou X, Wang Y, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q. Rapamycin and everolimus facilitate hepatitis E virus replication: revealing a basal defense mechanism of PI3K-PKB-mTOR pathway. J Hepatol. 2014;61:746–754. doi: 10.1016/j.jhep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Zhou X, Debing Y, Chen K, Van Der Laan LJ, Neyts J, Janssen HL, Metselaar HJ, Peppelenbosch MP, Pan Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146:1775–1783. doi: 10.1053/j.gastro.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 118.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, Muscari F, Izopet J. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 119.Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 120.Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. 1995;59:1426–1431. doi: 10.1097/00007890-199505270-00012. [DOI] [PubMed] [Google Scholar]
- 121.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 122.Kamar N, Abravanel F, Behrendt P, Hofmann J, Pageaux GP, Barbet C, Moal V, Couzi L, Horvatits T, De Man RA, Cassuto E, Elsharkawy AM, Riezebos-Brilman A, Scemla A, Hillaire S, Donnelly MC, Radenne S, Sayegh J, Garrouste C, Dumortier J, Glowaki F, Matignon M, Coilly A, Figueres L, Mousson C, Minello A, Dharancy S, Rerolle JP, Lebray P, Etienne I, Perrin P, Choi M, Marion O, Izopet J Hepatitis E Virus Ribavirin Study Group. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin Infect Dis. 2020;71:1204–1211. doi: 10.1093/cid/ciz953. [DOI] [PubMed] [Google Scholar]
- 123.Abravanel F, Lhomme S, Rostaing L, Kamar N, Izopet J. Protracted fecal shedding of HEV during ribavirin therapy predicts treatment relapse. Clin Infect Dis. 2015;60:96–99. doi: 10.1093/cid/ciu742. [DOI] [PubMed] [Google Scholar]
- 124.Kamar N, Lhomme S, Abravanel F, Cointault O, Esposito L, Cardeau-Desangles I, Del Bello A, Dörr G, Lavayssière L, Nogier MB, Guitard J, Ribes D, Goin AL, Broué P, Metsu D, Sauné K, Rostaing L, Izopet J. An Early Viral Response Predicts the Virological Response to Ribavirin in Hepatitis E Virus Organ Transplant Patients. Transplantation. 2015;99:2124–2131. doi: 10.1097/TP.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 125.Alric L, Bonnet D, Beynes-Rauzy O, Izopet J, Kamar N. Definitive clearance of a chronic hepatitis E virus infection with ribavirin treatment. Am J Gastroenterol. 2011;106:1562–1563. doi: 10.1038/ajg.2011.158. [DOI] [PubMed] [Google Scholar]
- 126.Neukam K, Barreiro P, Macías J, Avellón A, Cifuentes C, Martín-Carbonero L, Echevarría JM, Vargas J, Soriano V, Pineda JA. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis. 2013;57:465–468. doi: 10.1093/cid/cit224. [DOI] [PubMed] [Google Scholar]
- 127.Hajji H, Gérolami R, Solas C, Moreau J, Colson P. Chronic hepatitis E resolution in a human immunodeficiency virus (HIV)-infected patient treated with ribavirin. Int J Antimicrob Agents. 2013;41:595–597. doi: 10.1016/j.ijantimicag.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Tavitian S, Peron JM, Huguet F, Kamar N, Abravanel F, Beyne-Rauzy O, Oberic L, Faguer S, Alric L, Roussel M, Gaudin C, Ysebaert L, Huynh A, Recher C. Ribavirin for Chronic Hepatitis Prevention among Patients with Hematologic Malignancies. Emerg Infect Dis. 2015;21:1466–1469. doi: 10.3201/eid2108.150199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Debing Y, Emerson SU, Wang Y, Pan Q, Balzarini J, Dallmeier K, Neyts J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother. 2014;58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Debing Y, Gisa A, Dallmeier K, Pischke S, Bremer B, Manns M, Wedemeyer H, Suneetha PV, Neyts J. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology. 2014;147:1008–11.e7; quiz e15-16. doi: 10.1053/j.gastro.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 131.Debing Y, Ramière C, Dallmeier K, Piorkowski G, Trabaud MA, Lebossé F, Scholtès C, Roche M, Legras-Lachuer C, de Lamballerie X, André P, Neyts J. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65:499–508. doi: 10.1016/j.jhep.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 132.Galante A, Pischke S, Polywka S, Luetgehethmann M, Suneetha PV, Gisa A, Hiller J, Dienes HP, Nashan B, Lohse AW, Sterneck M. Relevance of chronic hepatitis E in liver transplant recipients: a real-life setting. Transpl Infect Dis. 2015;17:617–622. doi: 10.1111/tid.12411. [DOI] [PubMed] [Google Scholar]
- 133.Todt D, Gisa A, Radonic A, Nitsche A, Behrendt P, Suneetha PV, Pischke S, Bremer B, Brown RJ, Manns MP, Cornberg M, Bock CT, Steinmann E, Wedemeyer H. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut. 2016;65:1733–1743. doi: 10.1136/gutjnl-2015-311000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kamar N, Chatelut E, Manolis E, Lafont T, Izopet J, Rostaing L. Ribavirin pharmacokinetics in renal and liver transplant patients: evidence that it depends on renal function. Am J Kidney Dis. 2004;43:140–146. doi: 10.1053/j.ajkd.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 135.Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82–85.e4. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 136.Horvatits T, Schulze Zur Wiesch J, Lütgehetmann M, Lohse AW, Pischke S. The Clinical Perspective on Hepatitis E. Viruses. 2019;11 doi: 10.3390/v11070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Biliotti E, Franchi C, Spaziante M, Garbuglia AR, Volpicelli L, Palazzo D, De Angelis M, Esvan R, Taliani G. Autochthonous acute hepatitis E: treatment with sofosbuvir and ribavirin. Infection. 2018;46:725–727. doi: 10.1007/s15010-018-1168-7. [DOI] [PubMed] [Google Scholar]
- 138.Drinane M, Jing Wang X, Watt K. Sofosbuvir and Ribavirin Eradication of Refractory Hepatitis E in an Immunosuppressed Kidney Transplant Recipient. Hepatology. 2019;69:2297–2299. doi: 10.1002/hep.30428. [DOI] [PubMed] [Google Scholar]
- 139.Donnelly MC, Imlach SN, Abravanel F, Ramalingam S, Johannessen I, Petrik J, Fraser AR, Campbell JD, Bramley P, Dalton HR, Hayes PC, Kamar N, Simpson KJ. Sofosbuvir and Daclatasvir Anti-Viral Therapy Fails to Clear HEV Viremia and Restore Reactive T Cells in a HEV/HCV Co-Infected Liver Transplant Recipient. Gastroenterology. 2017;152:300–301. doi: 10.1053/j.gastro.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 140.Todesco E, Demeret S, Calin R, Roque-Afonso AM, Thibault V, Mallet V, Akhavan S, Jaspard M, Peytavin G, Poynard T, Katlama C, Pourcher V. Chronic hepatitis E in HIV/HBV coinfected patient: lack of power of sofosbuvir-ribavirin. AIDS. 2017;31:1346–1348. doi: 10.1097/QAD.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 141.van der Valk M, Zaaijer HL, Kater AP, Schinkel J. Sofosbuvir shows antiviral activity in a patient with chronic hepatitis E virus infection. J Hepatol. 2017;66:242–243. doi: 10.1016/j.jhep.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 142.Schulz M, Papp CP, Bock CT, Hofmann J, Gerlach UA, Maurer MM, Eurich D, Mueller T. Combination therapy of sofosbuvir and ribavirin fails to clear chronic hepatitis E infection in a multivisceral transplanted patient. J Hepatol. 2019;71:225–227. doi: 10.1016/j.jhep.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 143.Nishiyama T, Kobayashi T, Jirintai S, Nagashima S, Primadharsini PP, Nishizawa T, Okamoto H. Antiviral candidates against the hepatitis E virus (HEV) and their combinations inhibit HEV growth in in vitro. Antiviral Res. 2019;170:104570. doi: 10.1016/j.antiviral.2019.104570. [DOI] [PubMed] [Google Scholar]
- 144.Horvatits T, Schübel N, Kamar N, Polywka S, Lütgehetmann M, Rutter K, zur Wiesch JS, Manns MP, Lohse A, Roque-Afonso AM, Pischke S, Behrendt P, Mallet V. SAT-208-Zinc/Ribavirin: A possible treatment option in chronically HEV genotype 3 infected patients without SVR under ribavirin monotherapy. J Hepatol. 2019;70 Suppl:e721–e722. [Google Scholar]
- 145.Marion O, Abravanel F, Izopet J, Kamar N. Failure to respond to ribavirin despite elevated intra-erythrocyte zinc level in transplant-patients with chronic hepatitis E virus infection. Transpl Infect Dis. 2019;21:e13050. doi: 10.1111/tid.13050. [DOI] [PubMed] [Google Scholar]
- 146.Todt D, Moeller N, Praditya D, Kinast V, Friesland M, Engelmann M, Verhoye L, Sayed IM, Behrendt P, Dao Thi VL, Meuleman P, Steinmann E. The natural compound silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo. Antiviral Res. 2018;157:151–158. doi: 10.1016/j.antiviral.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Netzler NE, Enosi Tuipulotu D, Vasudevan SG, Mackenzie JM, White PA. Antiviral Candidates for Treating Hepatitis E Virus Infection. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.00003-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]