Abstract
When South Florida became a hot spot for COVID-19 disease in March 2020, we faced an urgent need to develop test capability to detect SARS-CoV-2 infection. We assembled a transdisciplinary team of knowledgeable and dedicated physicians, scientists, technologists, and administrators who rapidly built a multiplatform, polymerase chain reaction- and serology-based detection program, established drive-through facilities, and drafted and implemented guidelines that enabled efficient testing of our patients and employees. This process was extremely complex, due to the limited availability of needed reagents, but outreach to our research scientists and multiple diagnostic laboratory companies, and government officials enabled us to implement both Food and Drug Administration authorized and laboratory-developed testing–based testing protocols. We analyzed our workforce needs and created teams of appropriately skilled and certified workers to safely process patient samples and conduct SARS-CoV-2 testing and contact tracing. We initiated smart test ordering, interfaced all testing platforms with our electronic medical record, and went from zero testing capacity to testing hundreds of health care workers and patients daily, within 3 weeks. We believe our experience can inform the efforts of others when faced with a crisis situation.
Keywords: COVID-19, testing, academia, university, program
Introduction
The optimal response of an academic health system to a global health crisis requires sustained coordination between academic faculty across many departments and hospital and health system leadership to develop the policies and procedures, communication strategies, and teamwork needed for timely and effective decision-making. Novel crises place previously unidentifiable stressors across all aspects of an organization, and all weaknesses in the initial phases of a response must be rapidly identified and mitigated through clear policies and procedures that are easy to disseminate to all involved.
The first cases of COVID-19 disease occurred in Wuhan, China, and were reported globally by Chinese health authorities on December 31, 2019. While the first confirmed COVID-19 case in the United States was reported on January 21, 2020, the first case of COVID-19 disease in Florida was reported on March 1, 2020. At the time of this writing, Florida had 46 117 cases of COVID-19 disease, with 2096 deaths (as of May 20, 2020).1
The University of Miami Health System (UHealth) serves a 4-county catchment region with over 6 million inhabitants. We are the only University-based health system in the region, with 3 inpatient facilities and 7 satellite locations. The University of Miami’s Miller School of Medicine employs 1100 physicians, with a departmental structure as well as institutes and centers, including the Hussman Institute for Human Genomics and Sylvester Comprehensive Cancer Center.
In March 2020, with the spread of COVID-19 disease in major cities throughout the United States, we began COVID-19 testing by sending nasopharyngeal (NP) swab material to commercial laboratories for reverse transcription polymerase chain reaction (RT-PCR) testing. This proved problematic because of increasing turnaround times (TAT), so we began to formulate a plan for in-house COVID-19 testing. The Chief Clinical Officer and Interim Chief Operating Officer formed 4 leadership committees to address workforce management, clinical therapeutics, equipment, and resources and the Testing Committee, which was tasked with developing and implementing in-house testing and algorithms for testing patients and employees. Although our health care system has a longstanding and resilient emergency response infrastructure in place, based on extensive, annual preparations for the 6-month long hurricane season, preparation for the COVID-19 pandemic required the involvement of many additional personnel, with different skill sets, to address limited critical COVID-19 resources, develop and evolve testing capabilities and algorithms, while maintaining our standard of care for non-COVID-19 patients. Like other health systems, the widespread nature of the pandemic and the lethality of COVID-19 disease led us to postpone or cancel nonessential patient visits and surgeries and expand telemedicine capabilities. Social distancing, self-quarantine, and other preventative measures were instituted, which helped “flatten the curve.”
Public health surveillance and testing strategies are the cornerstone for effective COVID-19 evaluation and management, so we assembled a team of pathologists, infectious disease experts, scientists, hospital administrators, laboratory technicians, public health practitioners, and others who worked together to build a robust, multiplatform, PCR- and serology-based SARS-CoV-2 virus or antibody detection system. This system is being utilized to evaluate and monitor symptomatic patients, identify patients at risk, screen frontline employees, and assess those with a history of close contact with infected individuals. This system is enabling us to identify contagious individuals and those who may be immune. We have established contact tracing policies and procedures, implemented a COVID-19 convalescent plasma (CCP) program, and conducted Institutional Review Board (IRB)-approved clinical and translational research, including a program of community testing to define SARS-CoV-2 seroprevalence in our region.
Developing In-House Testing Capability
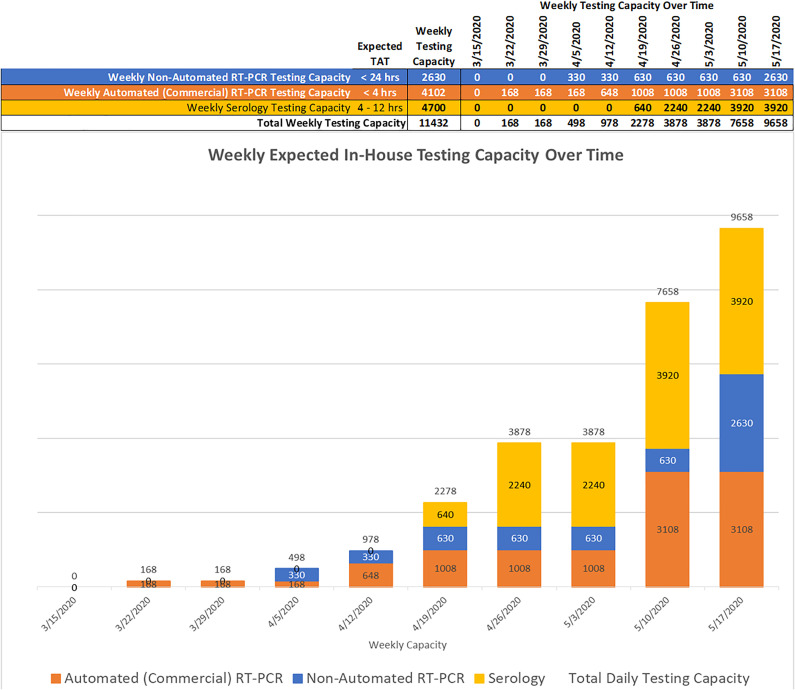
Our COVID-19 testing was initially outsourced to commercial reference laboratories. However, the prolonged TAT we soon experienced hindered our ability to adequately manage emergency department admissions and emergency procedures, among other activities, so we needed to rapidly develop in-house COVID-19 testing. We created 3 working groups that focused on implementing manual RT-PCR testing, automated/commercial PCR testing, and serologic testing. Each group was charged with developing diversified testing platforms, in order to achieve the flexibility needed to deal with supply chain issues, including shortages or delays in the availability of reagents or instrumentation, and the changing regulatory environment based on Food and Drug Administration (FDA) or other agency regulations and state policies.2 The group was able to build a new molecular laboratory designed for manual RT-PCR-based testing within 2 weeks, getting reagents and instruments from the basic scientists on the medical and marine school campuses. We then utilized 4 (CLIA)-certified laboratories for insourcing SARS-CoV-2 testing clinical laboratory improvement amendment, as shown in Table 1. These test procedures were chosen based on the in vitro diagnostic Emergency Use Authorizations (EUAs) published on the FDA website3 as well as available reagents and instrumentation. The testing platforms provide rapid TAT and varying but extensive test capacity (Table 1), which has been crucial to optimally manage specific groups of patients and increase capacity over time (Figure 1).
Table 1.
Testing Platforms, Turnaround Times, and Weekly Test Capacity.*
| Testing site | Test name | Instruments | TAT | Test capacity/week | |
|---|---|---|---|---|---|
| 2 | RT-PCR | Extraction: Qiacube |
PCR: 1. ABI7500 Fast or 2. QS6flex |
< 24 hrs | 600 |
| 3 | RT-PCR | Extraction: Chemagic 360 |
PCR: 1. ABI7500 or 2. QS6flex |
< 24 hrs | 1700 |
| 4 | RT-PCR | Extraction: Maxwell |
PCR: ABI 7500 |
< 24 hrs | 330 |
| Weekly Nonautomated RT-PCR testing capacity | 2630 | ||||
| 1 | RT-PCR | Eplex (GenMark) | < 4 hrs | 70† | |
| 1 | RT-PCR | Simplexa (DiaSorin #1) | < 4 hrs | 840 | |
| 1 | RT-PCR | Simplexa (DiaSorin #2) | < 4 hrs | 840 | |
| 1 | RT-PCR | Bio GX (BD Max) | < 4 hrs | 1260 | |
| 4 | RT-PCR | Ingenius (ELI Tech) | < 4 hrs | 672 | |
| Weekly automated (commercial) RT-PCR testing capacity | 4102 | ||||
| Total weekly RT-PCR testing capacity | 6732 | ||||
| 1 | LFD | BioMedomics/Cellex | < 4 hrs | 2240 | |
| 1 | ELISA | Dynext DS2 | < 12 hrs | 750 | |
| 4 | ELISA | Dynex DSX | < 12 hrs | 1500 | |
| Total weekly serology testing capacity | 4700 | ||||
Abbreviation: ELISA, enzyme-linked immunosorbent assays; LFD, lateral flow devices; RT-PCR, reverse transcription polymerase chain reaction.
* PCR and serologic testing platforms in place, listing the instruments, turnaround times (TAT), and test capacity per 7-day week, based on availability of reagents, personnel, and instrument capacity.
† Actual capacity of the GenMark machine is shown (5% of instrument capacity), reflecting limited availability of reagents.
Figure 1.
Weekly in-house testing capacity over time: Ramp up for weekly in-house COVID-19 testing shown, based on ordering additional reagents, hiring additional personnel, and the increased testing expected for patients and employees.
Despite its limitations, serologic testing serves as an important adjunct to PCR testing, given the possible false-negative rate of PCR-based testing for SARS-CoV-2 RNA4 and the need to detect evidence of prior infection and possible viral immunity. Several types of immunoassays were evaluated, including lateral flow devices (LFD), enzyme-linked immunosorbent assays (ELISA), and chemiluminescence immunoassays.
Different tests detect different antibody isotypes (IgM, IgG, IgA, or total antibody levels) directed against different targets (eg, the nucleocapsid, receptor binding domain, and spike proteins) and with differing sensitivity.5-9 Current analyses provide only a qualitative, positive or negative, result.
We validated the COVID-19 IgM/IgG Rapid Test kit, an LFD test from BioMedomics (Becton, Dickinson and Company), and the Epitope ELISA-based COVID-19 assay for detecting IgM and IgG at our CLIA-certified high-complexity laboratory. Using a venipuncture, serum or whole blood from inpatients was evaluated using the BioMedomics LFD test, which demonstrated 98% to 100% specificity and 71% to 73% sensitivity; these results were similar to the previously reported results of a larger validation cohort study (which reported 90.63% specificity and 88.66% sensitivity).10 The Epitope ELISA assay demonstrated specificity of 95% for IgM and 90% for IgG, with an overall sensitivity of 85% when compared to RT-PCR results for the same samples assayed by the BioMedomics LFD test. As of July 10, 2020, the Epitope IgG/IgM ELISA kit is still pending an FDA-EUA designation. The BioMedomics test was initially evaluated as an EUA assay, pending FDA approval, so we implemented it as a high-complexity laboratory-developed test (LDT). This required qualified laboratory personnel and a disclaimer in the result report stating that the test is not FDA approved. On May 7, 2020, the FDA removed this test from the EUA pending list, and hence, we immediately ceased using it for clinical testing. We subsequently acquired the Healgen FDA EUA-approved SARS-CoV-2 IgG/IgM rapid lateral flow immunoassay (LFIA); validation studies demonstrate good specificity of 100% for IgM and 97% for IgG and an overall sensitivity of 83%. Because this test does not require instrumentation, it could be utilized at our satellite locations as a point-of-care test. We also validated the FDA-EUA authorized Elecsys Roche Anti-SARS-CoV-2 total antibody test using the same samples as the ELISA and LFIA validation and found an overall specificity of 100% and sensitivity of 61%. This test could be a component of a high-throughput screening program, especially if reflexed to a test that can distinguish IgM from IgG antibody positivity.
The clinical applicability of SARS-CoV-2 serology testing is still under debate. According to both College of American Pathology and Centers for Disease Control and Prevention (CDC) recommendations and guidelines,11 serology should not be used to diagnose COVID-19 infection. However, positive serologic tests may help evaluate patients with suspected false-negative upper respiratory PCR testing. Serologic testing is important in identifying potential convalescent plasma donors and in evaluating the immune response to candidate vaccines.
We continue to encounter problems with reagent availability, especially those that have received FDA EUA approval, despite having assembled a team of project managers, industrial engineers, administrators, physicians, and scientists who along with the institution’s supply chain leaders and COVID-19 command center institutional team assure all requests for instruments and supplies are coordinated with industry and government agencies. We have also created interactive Gantt Charts that allow us to track the availability of supplies on a daily basis and assess staffing needs. Initially, we utilized medical technologists, who were working in research labs throughout the university and health system, who temporarily transitioned to work in our CLIA certified lab. We hired permanent staff, and have had to increase staffing, to accommodate the increased need for testing and to account for some attrition of staff, as the pandemic surges through our community.
We created a data mining and reporting team to ensure that institutional, state, and federal reporting data requirements are met. Key information reported included the number of tests performed each day, the percentage of positive results, up-to-date TAT for all laboratories, volume of orders by site and department, number of pending results, and number of rejected, not performed, or canceled tests, among others. We leveraged the electronic medical record and laboratory information system to capture information on the performing laboratory, the methodology and instrumentation used, the reference range, current procedural terminology codes for billing, results, and interpretation, and the required disclaimers stating the FDA status of EUAs or LDTs and the limitations of the tests.12-14
Community-Based Surveillance and Testing Initiatives
During the initial days of the pandemic, we worked with state, county, and city officials and agencies to support the establishment of community testing initiatives, helping establish best practices for the first drive-through testing site. Committee members toured the sites under development and provided nursing and other personnel to support the appointment scheduling lines. Working with Miami-Dade County (MDC) officials, we also established a surveillance program (see below) built upon random testing of cross sections of the county’s 2.8 million population using BioMedomics anti-SARS-CoV-2 antibody detection kits. We also provided surveillance and testing support to our first responders.
Employee Testing—Establishing a Robust Program
Call Center
A multidisciplinary group involving employee health, infection control, public health experts, and human resources devised a comprehensive algorithm for assessing returning travelers, as well as potential exposures within the community or the health care system. Our standard employee health processes and infrastructure were insufficient to manage the myriad of issues that the COVID-19 pandemic raised, requiring the development of a robust employee health call center capable of rapidly triaging the hundreds of calls received daily. We created a dedicated, centralized phone line that handled calls from employees to report positive test results, exposure to or potential symptoms of COVID-19, and was staffed by advanced practice registered nurses, registered nurses, and call center personnel. We established training procedures and algorithms that addressed employee testing, quarantine, and return to work policies to maintain a safe, essential health care workforce. Then, we began to order, schedule, and coordinate appointments for testing, providing testing instructions, communicate test results, and provide directions on employee return to work dispositions, including the need for quarantine. An epidemiology team was formed to conduct contact tracing on individuals with positive results and those who were presumptively positive but did not meet testing criteria (eg, employees who were able to complete work from home). These practices were approved by our Health Insurance Portability and Accountability Act (HIPAA), Privacy and Risk Management offices to ensure that employee data were appropriately managed. Later, we used the expertise of this staff to support other rapidly evolving needs, such as the testing and processing of potential donors for our CCP infusion research program. As of May 15, 2020, the call center had received 4925 calls and ordered 881 tests.
Drive-Through Testing Initiatives
To support the critical need for testing employees for COVID 19, we established a university-operated drive-through test site within 72 operational hours, capable of performing 84 NP swab tests per day, which uses a paperless check-in process that includes a 4- to 6-question questionnaire. The site was created to comply with all Authority Having Jurisdiction standards and use best practices to maintain staff and patient safety.
Employee Contact Tracing
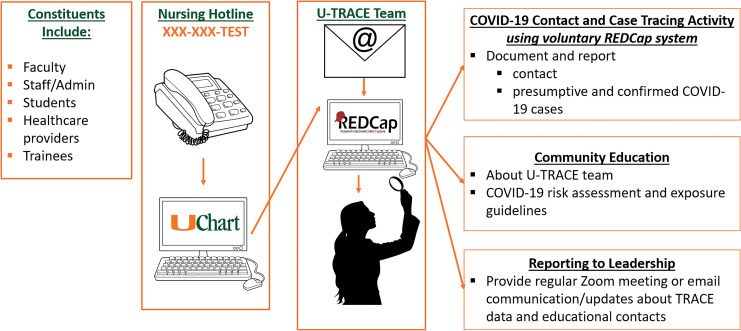
We also established a formal contact tracing program called UM Tracking, Resources, and Assessment of COVID-19 Epidemiology Program (U-TRACE), which engaged faculty with expertise in epidemiology, occupational health and safety, nursing, informatics, environmental exposure assessment, and public health education (Figure 2). To implement U-TRACE, we conducted a needs assessment of key stakeholder groups, designed the contact tracing workflow and data collection instruments, implemented a relational database to monitor and track COVID-19 cases, and adapted the program to federal, state, and local University/UHealth policy. This supported the coordination, tracking, and education of COVID-19 contact, presumptive, and confirmed cases among faculty, staff, and trainees. We used Research Electronic Data Capture (REDCap), a secure, HIPAA-compliant web-based application, to establish a relational database for our contact tracing, which allowed us to enter, review, or track COVID-19 persons under investigation, as well as COVID-19 cases among university and hospital employees. We established an employee hotline (XXX-XXX-TEST) to assist in contact tracing, which provided an initial clinical assessment of symptoms and triaged employees based on a risk assessment and separate return-to-work flow diagram.
Figure 2.
Contact Tracing Program: Contact tracing navigation is shown for faculty, staff, students, health care workers, and trainees.
Employees flagged for contact tracing were referred to the U-TRACE team by a telephone encounter note available in the electronic health record, by telephone or email. For each coworker identified by the employee (“patient zero”), the U-TRACE made 3 attempts to contact the coworker for referral and screening to the main employee hotline. To date, the U-TRACE program has completed 1681 employee encounters, of which 357 resulted in contact tracing due to COVID-19 presumed or confirmed positivity or positive COVID-19 symptoms. Approximately 81% of the employees who required contact tracing provided permission to let their coworkers know they were being evaluated as being potentially SARS-CoV-2 infected.
Employee Serologic Testing Initiative
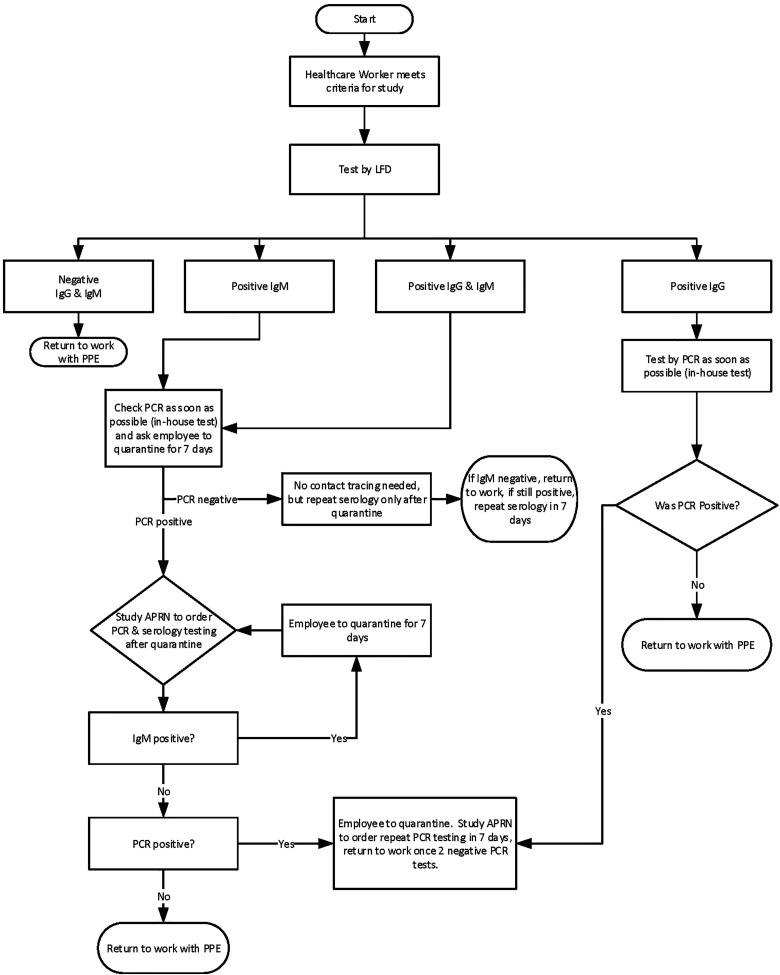
Health care workers (HCWs) are at high risk for SARS-CoV-2 infection and, especially if asymptomatic, could unknowingly transmit the virus to colleagues and/or patients. As part of our back-to-work strategic planning, to identify asymptomatic but possibly infected HCWs, we examined the utility of serologic testing to be reflexed to PCR testing where appropriate (Figure 3) using a UM IRB-approved research protocol (IRB: 20200506) and the BioMedomics Rapid IgM/IgG test that we internally validated. We enrolled 500 asymptomatic HCWs (out of over 1600 HCW volunteers) who were either working in clinic areas or the hospital and thus had potential exposure to COVID patient/samples over a 2- to 3-week period. The results of this study are currently being analyzed.
Figure 3.
Testing algorithm for asymptomatic health care workers, based on an IRB approved study of 500 employees, using serologic testing as the primary testing strategy, with reflex RT-PCR testing to determine whether employees with a positive serologic test have detectable SARS-CoV-2 virus.
Patient Testing Algorithms
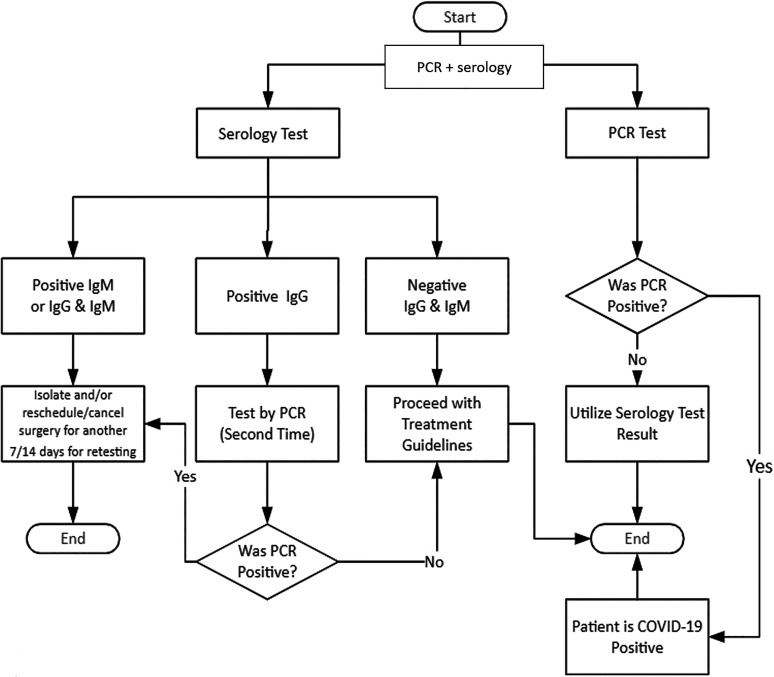
Our initial patient testing algorithms were based on the 3-tiered CDC priority system for testing patients with suspected SARS-CoV-2 infection.15 Limited access to required personal protective equipment,16 NP swabs, and varying TAT were the greatest constraints; thus, besides patient acuity, we incorporated the impact of utilization of scarce resources into our algorithm. Because our testing capabilities were rapidly expanding, our triage system needed to be flexible, so we also defined 3 phases of our SARS-CoV-2 testing program, with the first phase being the rollout phase. We did not define the second and third phases immediately nor provide a date or criteria that we would use to announce movement from one phase to another. The rollout phase focused on top priority cases including those being admitted to the hospital and those patients who required emergency interventions or a high-risk procedure (Figure 4). Our intent was to later include high-risk populations in the testing program, such as long-term facility residents and symptomatic patients older than 65 or with underlying health conditions. A team of clinicians and epidemiologists were involved in drafting these guidelines and in reviewing individual cases to approve prompt testing when a high likelihood of infection was suspected. Soon after we rolled out our patient testing algorithms, we began testing all admissions through the emergency department and all elective admissions for cancer care 48 to 72 hours prior to admission.
Figure 4.
Testing algorithm for asymptomatic patients undergoing high-risk procedures, involving a combination of PCR and serologic testing.
Established and Evolving COVID-19 Research Efforts
Community-Based Testing Program
Working with key administrators from the MDC Mayor’s Office and call center leadership from Florida Power and Light (FPL), we developed a community testing program called The Surveillance Project Assessing Risk and Knowledge of Coronavirus (SPARK-C) to approximate the seroprevalence of SARS-CoV-2 antibodies in MDC residents, determining the proportion of individuals infected, as well as their age, gender, and racial/ethnic distribution, their most common symptoms, and the fraction of asymptomatic infections. The FPL team randomly selected phone numbers to cover the geographic breadth of MDC, played an automated/commercial voice recording of the County Mayor, encouraged people answering the phone to participate, and then conducted antibody screening at a testing site nearest their home, typically a local park or library, after the selected individuals signed an informed consent form. Serologic testing was conducted using the BioMedomics serologic test. However, once the FDA removed this test from the EUA list, the SPARK-C program was temporarily paused to reconsider the best strategy for implementation.
Support for a Convalescent Plasma Infusion Program
We have established a CCP infusion program, as case series from China have shown promising results using convalescent plasma to treat patients with severe COVID-19.17-19 With our efforts in ramping up COVID-19 testing capability both for molecular testing and for serological testing, we established the following processes to support CCP collection: (1) created pathway to perform COVID-19 testing for potential CCP donors; (2) make COVID-19 testing results available for potential CCP donors; and (3) consent patients who require COVID-19 testing for future contact for potential CCP donation. To date, we have collected convalescent plasma, with assistance from OneBlood, from dozens of individuals.
Biospecimen Acquisition and Analyses
To promote multidisciplinary COVID-19 research, we have developed a COVID-19 clinically annotated biobank which contains consented and deidentified samples, including blood (serum, plasma, buffy coat samples) and cytology fluids, saliva, urine, fine needle aspirates, and surgical or autopsy tissue samples. Access to these materials will foster laboratory research, support novel clinical trials, and ideally generate data for National Institutes of Health and other grant proposals.
Conclusions
We activated a health system crisis response team in early March to tackle the unique challenges associated with the COVID-19 pandemic, including supply limitations (eg, NP swabs, collection kits, transport media, and regent kits) that were severely limiting our response, including our ability to perform PCR testing to detect SARS-CoV-2 virus. Necessary, but otherwise nondescript, products such as NP swabs, collection kits, transport media, and reagent kits were in acute shortage. Commercial laboratories and state health departments faced similar shortages and challenges and soon testing TAT ballooned to more than 5 to 7 days. We quickly realized the pitfalls of relying heavily on any single testing platform or vendor and created a testing committee charged with overseeing every aspect of testing. We pulled together resources, processes, expertise, reagents, machines, and decision-making tools from across the entire campus, including the academic leaders, the laboratory researchers, the operational leaders, and the procurement teams.
Together, we assembled numerous testing platforms, managed reagent inventory, communicated with external vendors, and developed testing algorithms for our patients and our employees. The strength of the university’s research enterprise enabled us to rapidly build up in-house testing capabilities, including serologic testing, which we ordered in bulk during the early phase of our testing efforts, with the goal of subsequently validating the assays in-house.
What is most evident from the current COVID-19 environment is the importance of planning, effective resourcing, nimble regulatory bodies, and clinical scientific rigor. To achieve these goals, we developed a leadership structure, assigned tasks to key individuals, evolved effective twice-daily communications via teleconferencing, and aligned accountability with responsibility. This was done in a rapidly changing environment, with different authorizations and approvals (mostly EUAs) coming from the FDA several times each week. Given the lead time to develop new testing platforms, we had to commit to certain workflows, even though improvements came along that necessitated changes in the reagents used, the instruments utilized, or the body fluids subjected to analysis.
The role of accessible diagnostics is “front and center” in this crisis—not only for knowing whether someone has the virus but also whether they may be immune to subsequent infection or a potential donor for our CCP donation program. At the time of submitting the galley proofs, we have done approximately 39,000 RT-PCR tests and our hotline has answered over 13,800 calls.
We hope our experience in bringing together health care personnel from the health system, the university’s research community, and its administrative leadership can instruct other health care systems rapidly ramp up their ability to test for pathogens and implement health care policies that enable them to effectively respond to a health crisis.
Acknowledgments
The authors thank the many dedicated health care personnel who have worked under difficult conditions to keep our patients and employees safe. The authors also thank many members of UM, UHealth, and our community for assisting in establishing and maintaining our COVID-19 testing and surveillance program.
Authors’ Note: S.D.N., M.J., and D.P. contributed to the conceptualization of the manuscript, S.D.N. and M.J. to the organization, editing, and final drafting of the document. J.C., L.R., A.A., Y.W., S.W., L.P., L.G., M.G.B., C.C., J.M., M.S., G.C., M.M., B.H., A.C.M., E.K. contributed to operational and technical aspects of the work described in this report, to drafting the document, and final approval. D.A., Y.S.F., Y.Z., O.M., M.T., B.V., M.T., J.M., P.R., P.P., S.P., M.P., M.L., contributed to operational and technical aspects of the work described in this report and to final approval. All authors agree to be held accountable for all aspects of the work related to its accuracy and integrity.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The University of Miami entered into a management agreement with Lab Corp on May 3, 2020, with Lab Corp agreeing to provide management for a broad range of clinical laboratory tests conducted on UM in-patients. M.M. is a member of the Viracor Eurofins medical advisory board.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alvaro Alencar  https://orcid.org/0000-0002-1720-9295
https://orcid.org/0000-0002-1720-9295
Alberto Caban-Martinez  https://orcid.org/0000-0002-5960-1308
https://orcid.org/0000-0002-5960-1308
References
- 1. Florida Covid-19 Response. Florida Department of Health. 2020. Accessed August 15, 2020 https://floridahealthcovid19.gov.
- 2. Public Readiness and Emergency Preparedness Act (PREP Act), US Department of Health and Human Services. 2020. Accessed August 15, 2020. https://www.phe.gov/Preparedness/Pages/default.aspx
- 3. Emergency Use Authorizations for Medical Devices. US Food and Drug Administration. 2020. Accessed August 15, 2020 https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- 4. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020; 323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med. 2020;172:726–734. doi:10.7326/M20-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo L, Ren L, Yang S. et al . Profiling early humoral response to diagnose novel coronavirus diseases (COVID-19). Clin Infect Dis. 2020;71:778–785.doi:10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer B, Drosten C, Muller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Research. 2014;194:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okba NMA, Müller MA, Li W, et al. Sever acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26:1478–1488.doi:10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 28, 2020]. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis [published online February 27, 2020]. J Med Virol. 2020. doi:10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Using Antibody Tests for COVID-19. Centers for Disease Control and Prevention. 2020. Accessed August 15, 2020 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html. [PubMed]
- 12. Cockburn A. Agile software development joins the “Would-Be” crowd. Cut IT J. 2002;15:6–12. [Google Scholar]
- 13. Cooke NJ, Hilton ML. eds. Enhancing the Effectiveness of Team Science. Washington (D.C) Nat Academies Press USA; 2015. [PubMed] [Google Scholar]
- 14. Fernandez DJ, Fernandez JD. Agile project management—agilism versus traditional approaches. J Comput Inf Syst. 2008; 49:10–17. [Google Scholar]
- 15. Preparedness Tools for Healthcare Professionals and Facilities Responding to Coronavirus (COVID-19). Centers for Disease Control and Prevention. 2020. Accessed August 15, 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/preparedness-checklists.html
- 16. Hanna TP, Evans GA, Booth CM. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020; 17:268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020; 323:1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020; 117:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China [published online April 15, 2020]. J Med Virol. 2020. doi:10.1002/jmv.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]