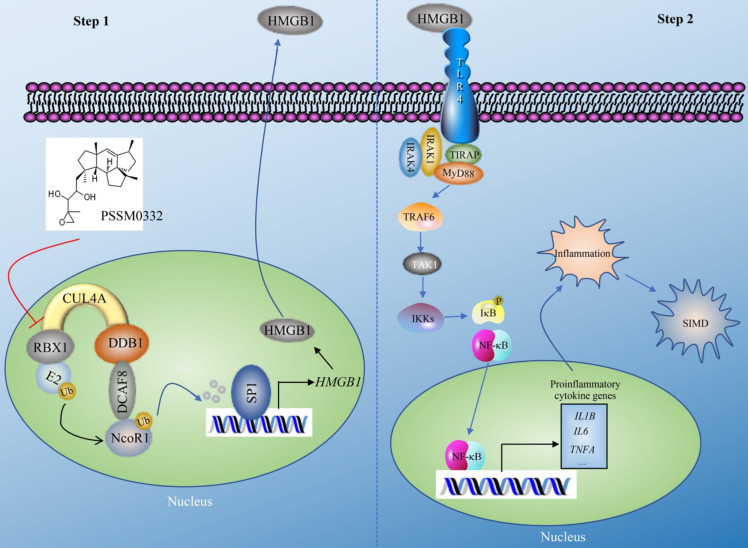
Figure 8.
A representative model of CRL4ADCAF8 E3 ligase-mediated signaling in the pathogenesis of SIMD. CRL4ADCAF8 E3 ligase-mediated signaling in the pathogenesis of SIMD can be divided into two steps. In the first step, CUL4A associates with RBX1, DDB1 and DCAF8 to form the CRL4ADCAF8 E3 ligase complex, which recognizes NcoR1 as a substrate, leading to degradation in the pathogenesis of SIMD. The degraded NcoR1 cannot function effectively as a corepressor to inhibit SP1-mediated transcription, causing the upregulation of HMGB1. In the second step, mature HMGB1 in the extracellular membrane binds to TLR4 to initiate downstream events, leading to the activation of a cascade that includes TIRAP, MyD88, IRAK1, IRAK4, TRAF6, TAK1 and IKKs. The phosphorylation of IκB mediated by IKKs impairs its inhibition of NF-κB, leading to the release and translocation of NF-κB. In the nucleus, NF-κB induces the expression of proinflammatory genes and results in an inflammatory response, leading to the pathogenesis of SIMD. The small molecule PSSM0332 specifically disrupts the CUL4A-RBX1 interaction, impairing the assembly of the CRL4DCAF8 E3 ligase complex and affecting downstream events, eventually improving the inflammatory response and alleviating the SIMD outcome.